Page 152 - Principles of Catalyst Development
P. 152
140 CHAPTER 7
a
N,(OH), DECOMPOSITION
5r
I
~ 10 1-
U)
U)
0 15 r
-.J
f-
I
Q 20 I
w
~ i
25t
30
100 200 300 400 500 600 700
TEMPERA TURE (0 C)
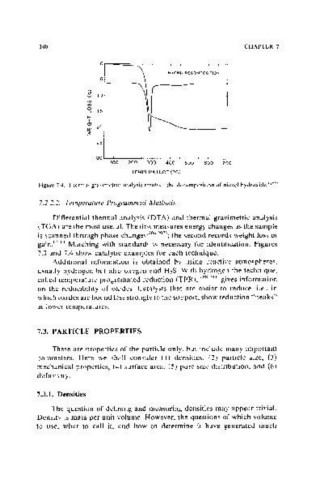
Figure 7.4. Thermal gravimetric analysis results-the decomposition of nickel hydroxide. II021
7.2.2.2. Temperature Programmed Methods
Differential thermal analysis (DT A) and thermal gravimetric analysis
(TGA) are the most useful. The first measures energy changes as the sample
is scanned through phase changes(206,207); the second records weight loss or
gain.(206) Matching with standards is necessary for identification. Figures
7.3 and 7.4 show catalytic examples for each technique.
Additional information is obtained by using reactive atmospheres,
usually hydrogen but also oxygen and H 2S. With hydrogen the technique,
called temperature programmed reduction (TPR)/20b,209) gives information
on the reducibility of oxides. Catalysts that are easier to reduce, i.e., in
which oxides are bound less strongly to the support, show reduction "peaks"
at lower temperatures.
7.3. PARTICLE PROPERTIES
These are properties of the particle only, but include many important
parameters. Here we shall consider (1) densities, (2) particle size, (3)
mechanical properties, (4) surface area, (5) pore size distribution, and (6)
diffusivity.
7.3.1. Densities
The question of defining and measuring densities may appear trivial.
Density is mass per unit volume. However, the questions of which volume
to use, what to call it, and how to determine it have generated much