Page 220 - Principles of Catalyst Development
P. 220
CATALYST DEACTIVATION 209
These poisons are removed during regenerative burning but may cause
problems by producing NO x in combustion effluents. It is better to remove
them before the process. Hydrotreating removes nitrogen, and this is stan-
dard procedure with all catalytic cracking feedstocks. This treatment also
removes sulfur and much of the heavy metal contamination.
8.3.7.4. Beneficial Poisons
Poisons generally result in harmful effects such as loss of activity, yet
there are circumstances when small amounts of poison are beneficial. An
example of this is catalytic reforming to boost the octane number of
paraffinic naphthas. Early workers noticed that a few parts per million of
sulfur in the feed increased reformate yield rather than decreasing as
expected. This "promotion" technique has become standard in reforming
technology. (277) The reason for this is seen in studies with pure compounds
on nickel-faujasite catalysts. (246) Nickel results in more hydrogenolysis than
platinum. This reaction leads to light gases and coke and is undesirable
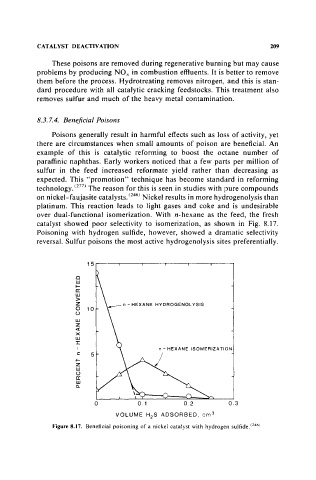
over dual-functional isomerization. With n-hexane as the feed, the fresh
catalyst showed poor selectivity to isomerization, as shown in Fig. 8.17.
Poisoning with hydrogen sulfide, however, showed a dramatic selectivity
reversal. Sulfur poisons the most active hydrogenolysis sites preferentially.
15
Cl
....
W
a:
w
>
z
0 10 ___ n - HEX ANE HYDROGENOL YSIS
()
w
Z
<l:
X
w
J:
n - HEXANE ISOMERIZATION
c 5
....
z
UJ
()
a:
w
c-
O 0.1 0.2 0.3
VOLUME H 2 S ADSORBED, cm 3
Figure 8.17. Beneficial poisoning of a nickel catalyst with hydrogen sulfide.(246)