Page 262 - Principles of Catalyst Development
P. 262
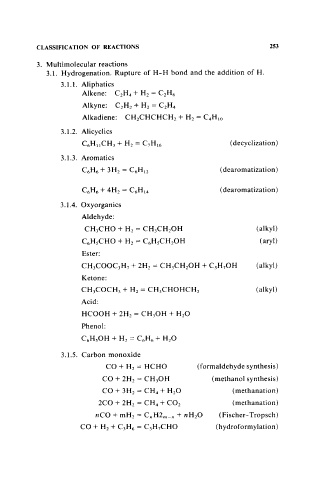
CLASSIFICATION OF REACTIONS 253
3. MuItimolecular reactions
3.1. Hydrogenation. Rupture of H-H bond and the addition of H.
3.1.1. Aliphatics
Alkene: C2H 4 + H2 = C2H6
Alkyne: C2H2 + H2 = C2H 4
Alkadiene: CH2CHCHCH2 + H2 = C 4H lO
3.1.2. Alicyclics
C6H II CH 3 + H2 = C 7H 16 ( decyclization)
3.1.3. Aromatics
C6H6 + 3H2 = C6Hl2 (dearomatization)
(dearomatization)
3.1.4. Oxyorganics
Aldehyde:
CH 3CHO + H2 = CH 3CH20H (alkyl)
C6HsCHO + H2 = C 6HsCH 20H ( aryl)
Ester:
CH 3COOC 3H 7 + 2H2 = CH 3CH2 0H + C 3H 70H (alkyl)
Ketone:
(alkyl)
CH3 COCH 3 + H2 = CH 3CHOHCH 3
Acid:
HCOOH + 2H2 = CH 30H + H20
Phenol:
C6HsOH + H2 = C6H6 + H 20
3.1.5. Carbon monoxide
CO+ H2 = HCHO (formaldehyde synthesis)
CO + 2H2 = CH 30H (methanol synthesis)
CO + 3H2 = CH 4 + H2 0 ( methanation)
2CO + 2H2 = CH 4 + CO2 (methanation)
nCO + mH2 = CnH2m-n + nH 20 (Fischer-Tropsch)
CO + H2 + C3H6 = C 3H 7CHO (hydroformylation)