Page 52 - Principles of Catalyst Development
P. 52
38 CHAPTER 2
complexity, from fixed to trickle bed reactors and even ebullating processes.
Catalyst particles are available in a wide range of sizes and shapes to fill
these needs. (70)
The fundamental chemistry, however, remains the same. The preferred
support is 'Y-AI203 prepared by precipitation and preformed into particles
with designed pore size, shapes, and distribution. Details are given in
2
Chapter 6. High surface area (~250 m g -I) is achieved during preparation.
Small amounts (~1 %) of SiO~ are added to stabilize the high area phase.
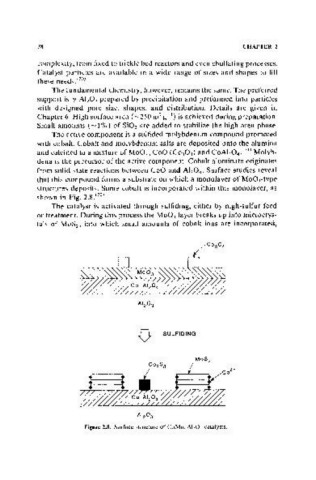
The active component is a sulfided molybdenum compound promoted
with cobalt. Cobalt and molybdenum salts are deposited onto the alumina
and calcined to a mixture of MoO), CoO (C0 30 4 ) and CoAl 20 4 YI) Molyb-
dena is the precursor of the active component. Cobalt aluminate originates
from solid state reactions between CoO and A1 20 4 • Surface studies reveal
that this compound forms a substrate on which a monolayer of MoOrtype
structures deposits. Some cobalt is incorporated within this monolayer, as
shown in Fig. 2.8. (72)
The catalyst is activated through sulfiding, either by high-sulfur feed
or treatment. During this process the Mo0 3 layer breaks up into microcrys-
tals of MoS 2 , into which small amounts of cobalt ions are incorporated,
~-
/
Q SULFIDING
2+
o
Figure 2.8. Surface structure of CoMo/ Al 20, catalysts.