Page 210 - Radiochemistry and nuclear chemistry
P. 210
194 Radiochemistry and Nuclear Chemistry
~ CAMERA
aaaaaaamaaaaaaac~,.
~]
' ~" '" "' 'c~";- CLASS top
"*-- RUBBER
LIGHT BEAM =~'~" T~ ~P"OI~TED BASF~
' Ol APIiP.AGM
VALVs
9 .
(~~ VACUUII
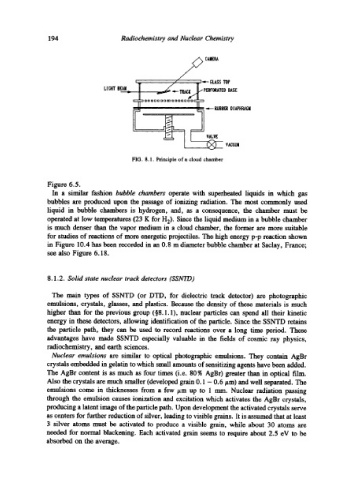
FIG. 8.1. Principle of a cloud chamber
Figure 6.5.
In a similar fashion bubble chambers operate with superheated liquids in which gas
bubbles are produced upon the passage of ionizing radiation. The most commonly used
liquid in bubble chambers is hydrogen, and, as a consequence, the chamber must be
operated at low temperatures (23 K for H2). Since the liquid medium in a bubble chamber
is much denser than the vapor medium in a cloud chamber, the former are more suitable
for studies of reactions of more energetic projectiles. The high energy p-p reaction shown
in Figure 10.4 has been recorded in an 0.8 m diameter bubble chamber at Saclay, France;
see also Figure 6.18.
8.1.2. Solid state nuclear track detectors (SSNTD)
The main types of SSNTD (or DTD, for dielectric track detector) are photographic
emulsions, crystals, glasses, and plastics. Because the density of these materials is much
higher than for the previous group (w nuclear particles can spend all their kinetic
energy in these detectors, allowing identification of the particle. Since the SSNTD retains
the particle path, they can be used to record reactions over a long time period. These
advantages have made SSNTD especially valuable in the fields of cosmic ray physics,
radiochemistry, and earth sciences.
Nuclear emulsions are similar to optical photographic emulsions. They contain AgBr
crystals embedded in gelatin to which small amounts of sensitizing agents have been added.
The AgBr content is as much as four times (i.e. 80% AgBr) greater than in optical film.
Also the crystals are much smaller (developed grain 0.1 - 0.6 #m) and well separated. The
emulsions come in thicknesses from a few/xm up to 1 ram. Nuclear radiation passing
through the emulsion causes ionization and excitation which activates the AgBr crystals,
producing a latent image of the particle path. Upon development the activated crystals serve
as centers for further reduction of silver, leading to visible grains. It is assumed that at least
3 silver atoms must be activated to produce a visible grain, while about 30 atoms are
needed for normal blackening. Each activated grain seems to require about 2.5 eV to be
absorbed on the average.