Page 65 - Radiochemistry and nuclear chemistry
P. 65
54 Radiochemistry and Nuclear Chemistry
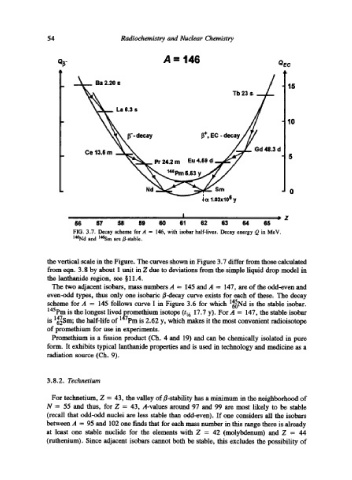
A= 146 QEC
Ba 2.20 s
15
Tb 23 s,
La 6.3 s
10
p'-decay J~+, EC - decay
Ce 13.6 m Gd 48.3 d
u
Pr24.2m Eu4.59d
146pnl 6.63 y
Nd Sm " 0
r 1.03x106 y
I "-Z
$6 57 $8 $9 60 61 62 63 64 65
FIG. 3.7. Decay scheme for A = 146, with isobar half-lives. Decay energy Q in MeV.
146Nd and lae'Sm are ~-stable.
the vertical scale in the Figure. The curves shown in Figure 3.7 differ from those calculated
from eqn. 3.8 by about 1 unit in Z due to deviations from the simple liquid drop model in
the lanthanide region, see w
The two adjacent isobars, mass numbers A = 145 and A = 147, are of the odd-even and
even-odd types, thus only one isobaric B-decay curve exists for each of these. The decay
scheme for A = 145 follows curve I in Figure 3.6 for which 145~ra is the stable isobar.
60" 9 "
145pm is the longest livedpromethium isotope (tt,~ 17.7 y). For A = 147, the stable isobar
147
is 62Sm; the half-life of 147 Pm is 2.62 y, which makes it the most convenient radioisotope
of promethium for use in experiments.
Promethium is a fission product (Ch. 4 and 19) and can be chemically isolated in pure
form. It exhibits typical lanthanide properties and is used in technology and medicine as a
radiation source (Ch. 9).
3.8.2. Technetium
For technetium, Z = 43, the valley of B-stability has a minimum in the neighborhood of
N = 55 and thus, for Z = 43, A-values around 97 and 99 are most likely to be stable
(recall that odd-odd nuclei are less stable than odd-even). If one considers all the isobars
between A = 95 and 102 one finds that for each mass number in this range there is already
at least one stable nuclide for the elements with Z = 42 (molybdenum) and Z = 44
(ruthenium). Since adjacent isobars cannot both be stable, this excludes the possibility of