Page 484 - Refining Biomass Residues for Sustainable Energy and Bioproducts
P. 484
Processing food waste for the production of platform chemicals 441
DHMF
Monosaccharides Mineral acids, polar
solvent, heteropolyacids, LA
ionic liquids
Dehydration HMF HMTHFA
Disaccharides
Hydrolysis Liquid alkanes
Polysaccharides FDCA
BrØnsted acids HFCA
(sulfuric, hydrochloric,
Amberlyst)
Termoset resins Polyurethanes Polyesters Polyamides
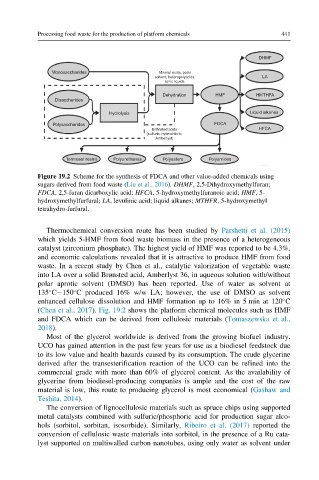
Figure 19.2 Scheme for the synthesis of FDCA and other value-added chemicals using
sugars derived from food waste (Liu et al., 2016). DHMF, 2,5-Dihydroxymethylfuran;
FDCA, 2,5-furan dicarboxylic acid; HFCA, 5-hydroxymethylfuranoic acid; HMF,5-
hydroxymethylfurfural; LA, levulinic acid; liquid alkanes; MTHFR, 5-hydroxymethyl
tetrahydro-furfural.
Thermochemical conversion route has been studied by Parshetti et al. (2015)
which yields 5-HMF from food waste biomass in the presence of a heterogeneous
catalyst (zirconium phosphate). The highest yield of HMF was reported to be 4.3%,
and economic calculations revealed that it is attractive to produce HMF from food
waste. In a recent study by Chen et al., catalytic valorization of vegetable waste
into LA over a solid Brønsted acid, Amberlyst 36, in aqueous solution with/without
polar aprotic solvent (DMSO) has been reported. Use of water as solvent at
135 C 150 C produced 16% w/w LA; however, the use of DMSO as solvent
enhanced cellulose dissolution and HMF formation up to 16% in 5 min at 120 C
(Chen et al., 2017). Fig. 19.2 shows the platform chemical molecules such as HMF
and FDCA which can be derived from cellulosic materials (Tomaszewska et al.,
2018).
Most of the glycerol worldwide is derived from the growing biofuel industry.
UCO has gained attention in the past few years for use as a biodiesel feedstock due
to its low value and health hazards caused by its consumption. The crude glycerine
derived after the transesterification reaction of the UCO can be refined into the
commercial grade with more than 60% of glycerol content. As the availability of
glycerine from biodiesel-producing companies is ample and the cost of the raw
material is low, this route to producing glycerol is most economical (Gashaw and
Teshita, 2014).
The conversion of lignocellulosic materials such as spruce chips using supported
metal catalysts combined with sulfuric/phosphoric acid for production sugar alco-
hols (sorbitol, sorbitan, isosorbide). Similarly, Ribeiro et al. (2017) reported the
conversion of cellulosic waste materials into sorbitol, in the presence of a Ru cata-
lyst supported on multiwalled carbon nanotubes, using only water as solvent under