Page 164 - Safety Risk Management for Medical Devices
P. 164
Software Risk Management 143
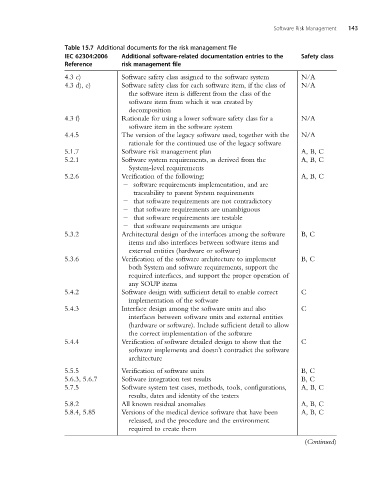
Table 15.7 Additional documents for the risk management file
IEC 62304:2006 Additional software-related documentation entries to the Safety class
Reference risk management file
4.3 c) Software safety class assigned to the software system N/A
4.3 d), e) Software safety class for each software item, if the class of N/A
the software item is different from the class of the
software item from which it was created by
decomposition
4.3 f) Rationale for using a lower software safety class for a N/A
software item in the software system
4.4.5 The version of the legacy software used, together with the N/A
rationale for the continued use of the legacy software
5.1.7 Software risk management plan A, B, C
5.2.1 Software system requirements, as derived from the A, B, C
System-level requirements
5.2.6 Verification of the following: A, B, C
2 software requirements implementation, and are
traceability to parent System requirements
2 that software requirements are not contradictory
2 that software requirements are unambiguous
2 that software requirements are testable
2 that software requirements are unique
5.3.2 Architectural design of the interfaces among the software B, C
items and also interfaces between software items and
external entities (hardware or software)
5.3.6 Verification of the software architecture to implement B, C
both System and software requirements, support the
required interfaces, and support the proper operation of
any SOUP items
5.4.2 Software design with sufficient detail to enable correct C
implementation of the software
5.4.3 Interface design among the software units and also C
interfaces between software units and external entities
(hardware or software). Include sufficient detail to allow
the correct implementation of the software
5.4.4 Verification of software detailed design to show that the C
software implements and doesn’t contradict the software
architecture
5.5.5 Verification of software units B, C
5.6.3, 5.6.7 Software integration test results B, C
5.7.5 Software system test cases, methods, tools, configurations, A, B, C
results, dates and identity of the testers
5.8.2 All known residual anomalies A, B, C
5.8.4, 5.85 Versions of the medical device software that have been A, B, C
released, and the procedure and the environment
required to create them
(Continued)