Page 80 - Safety Risk Management for Medical Devices
P. 80
Risk Management Process 59
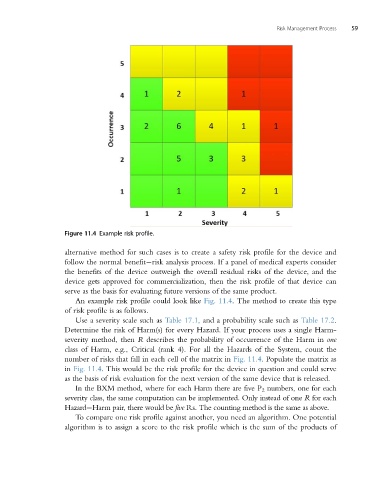
Figure 11.4 Example risk profile.
alternative method for such cases is to create a safety risk profile for the device and
follow the normal benefit risk analysis process. If a panel of medical experts consider
the benefits of the device outweigh the overall residual risks of the device, and the
device gets approved for commercialization, then the risk profile of that device can
serve as the basis for evaluating future versions of the same product.
An example risk profile could look like Fig. 11.4. The method to create this type
of risk profile is as follows.
Use a severity scale such as Table 17.1, and a probability scale such as Table 17.2.
Determine the risk of Harm(s) for every Hazard. If your process uses a single Harm-
severity method, then R describes the probability of occurrence of the Harm in one
class of Harm, e.g., Critical (rank 4). For all the Hazards of the System, count the
number of risks that fall in each cell of the matrix in Fig. 11.4. Populate the matrix as
in Fig. 11.4. This would be the risk profile for the device in question and could serve
as the basis of risk evaluation for the next version of the same device that is released.
In the BXM method, where for each Harm there are five P 2 numbers, one for each
severity class, the same computation can be implemented. Only instead of one R for each
Hazard Harm pair, there would be five Rs. The counting method is the same as above.
To compare one risk profile against another, you need an algorithm. One potential
algorithm is to assign a score to the risk profile which is the sum of the products of