Page 61 - Theory and Problems of BEGINNING CHEMISTRY
P. 61
50 ATOMS AND ATOMIC MASSES [CHAP. 3
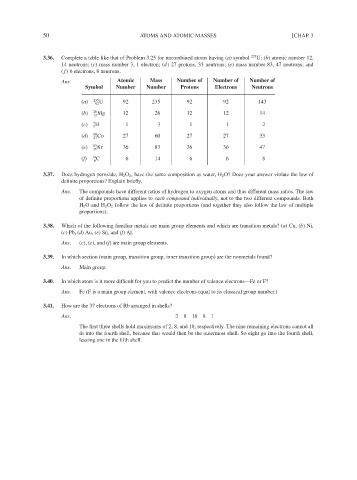
3.36. Complete a table like that of Problem 3.25 for uncombined atoms having (a) symbol 235 U; (b) atomic number 12,
14 neutrons; (c) mass number 3, 1 electron; (d) 27 protons, 33 neutrons; (e) mass number 83, 47 neutrons; and
( f ) 6 electrons, 8 neutrons.
Ans. Atomic Mass Number of Number of Number of
Symbol Number Number Protons Electrons Neutrons
(a) 235 U 92 235 92 92 143
92
(b) 26 Mg 12 26 12 12 14
12
(c) 3 1 H 1 3 1 1 2
(d) 60 Co 27 60 27 27 33
27
(e) 83 Kr 36 83 36 36 47
36
(f) 14 C 6 14 6 6 8
6
3.37. Does hydrogen peroxide, H 2 O 2 , have the same composition as water, H 2 O? Does your answer violate the law of
definite proportions? Explain briefly.
Ans. The compounds have different ratios of hydrogen to oxygen atoms and thus different mass ratios. The law
of definite proportions applies to each compound individually, not to the two different compounds. Both
H 2 O and H 2 O 2 follow the law of definite proportions (and together they also follow the law of multiple
proportions).
3.38. Which of the following familiar metals are main group elements and which are transition metals? (a) Cu, (b) Ni,
(c) Pb, (d) Au, (e) Sn, and (f) Al.
Ans. (c), (e), and (f) are main group elements.
3.39. In which section (main group, transition group, inner transition group) are the nonmetals found?
Ans. Main group.
3.40. In which atom is it more difficult for you to predict the number of valence electrons—Fe or F?
Ans. Fe (F is a main group element, with valence electrons equal to its classical group number.)
3.41. How are the 37 electrons of Rb arranged in shells?
Ans. 281881
The first three shells hold maximums of 2, 8, and 18, respectively. The nine remaining electrons cannot all
fit into the fourth shell, because that would then be the outermost shell. So eight go into the fourth shell,
leaving one in the fifth shell.