Page 176 - Theory and Problems of BEGINNING CHEMISTRY
P. 176
CHAP. 11] MOLARITY 165
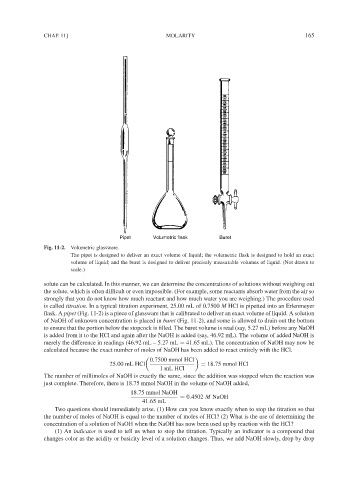
Pipet Volumetric flask Buret
Fig. 11-2. Volumetric glassware.
The pipet is designed to deliver an exact volume of liquid; the volumetric flask is designed to hold an exact
volume of liquid; and the buret is designed to deliver precisely measurable volumes of liquid. (Not drawn to
scale.)
solute can be calculated. In this manner, we can determine the concentrations of solutions without weighing out
the solute, which is often difficult or even impossible. (For example, some reactants absorb water from the air so
strongly that you do not know how much reactant and how much water you are weighing.) The procedure used
is called titration. In a typical titration experiment, 25.00 mL of 0.7500 M HCl is pipetted into an Erlenmeyer
flask. A pipet (Fig. 11-2) is a piece of glassware that is calibrated to deliver an exact volume of liquid. A solution
of NaOH of unknown concentration is placed in buret (Fig. 11-2), and some is allowed to drain out the bottom
to ensure that the portion below the stopcock is filled. The buret volume is read (say, 5.27 mL) before any NaOH
is added from it to the HCl and again after the NaOH is added (say, 46.92 mL). The volume of added NaOH is
merely the difference in readings (46.92 mL – 5.27 mL = 41.65 mL). The concentration of NaOH may now be
calculated because the exact number of moles of NaOH has been added to react entirely with the HCl.
0.7500 mmol HCl
25.00 mL HCl = 18.75 mmol HCl
1mLHCl
The number of millimoles of NaOH is exactly the same, since the addition was stopped when the reaction was
just complete. Therefore, there is 18.75 mmol NaOH in the volume of NaOH added,
18.75 mmol NaOH
= 0.4502 M NaOH
41.65 mL
Two questions should immediately arise. (1) How can you know exactly when to stop the titration so that
the number of moles of NaOH is equal to the number of moles of HCl? (2) What is the use of determining the
concentration of a solution of NaOH when the NaOH has now been used up by reaction with the HCl?
(1) An indicator is used to tell us when to stop the titration. Typically an indicator is a compound that
changes color as the acidity or basicity level of a solution changes. Thus, we add NaOH slowly, drop by drop