Page 256 - Theory and Problems of BEGINNING CHEMISTRY
P. 256
CHAP. 16] RATES AND EQUILIBRIUM 245
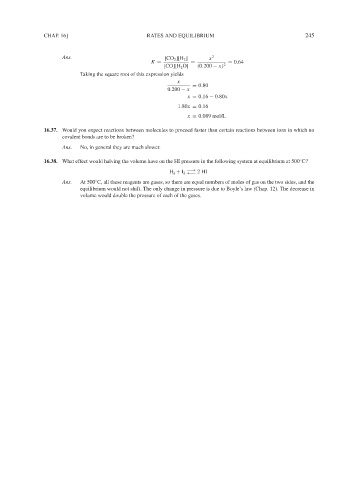
Ans. [CO 2 ][H 2 ] x 2
K = = = 0.64
[CO][H 2 O] (0.200 − x) 2
Taking the square root of this expression yields
x
= 0.80
0.200 − x
x = 0.16 − 0.80x
1.80x = 0.16
x = 0.089 mol/L
16.37. Would you expect reactions between molecules to proceed faster than certain reactions between ions in which no
covalent bonds are to be broken?
Ans. No, in general they are much slower.
◦
16.38. What effect would halving the volume have on the HI pressure in the following system at equilibrium at 500 C?
−→
H 2 + I 2 ←− 2HI
Ans. At 500 C, all these reagents are gases, so there are equal numbers of moles of gas on the two sides, and the
◦
equilibrium would not shift. The only change in pressure is due to Boyle’s law (Chap. 12). The decrease in
volume would double the pressure of each of the gases.