Page 111 - Separation process principles 2
P. 111
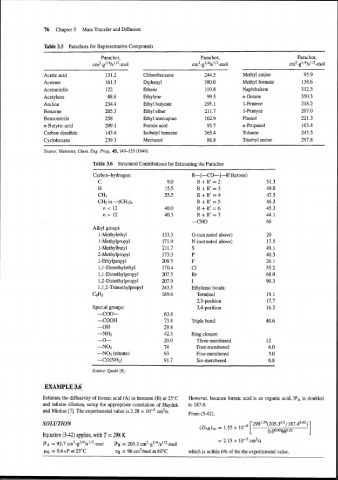
76 Chapter 3 Mass Transfer and Diffusion
Table 3.5 Parachors for Representative Compounds
Parachor, Parachor, Parachor,
~m~-~~/~/s'/~-mol ~m~-~'/~/s'/~-mol ~m~-~'/~/s'/~-mol
Acetic acid 131.2 Chlorobenzene 244.5 Methyl amine 95.9
Acetone 161.5 Diphenyl 380.0 Methyl formate 138.6
Acetonitrile 122 Ethane 110.8 Naphthalene 312.5
Acetylene 88.6 Ethylene 99.5 n-Octane 350.3
Aniline 234.4 Ethyl butyrate 295.1 1-Pentene 218.2
Benzene 205.3 Ethyl ether 211.7 1-Pentyne 207.0
Benzonitrile 25 8 Ethyl mercaptan 162.9 Phenol 221.3
n-Butyric acid 209.1 Formic acid 93.7 n-Propanol 165.4
Carbon disulfide 143.6 Isobutyl benzene 365.4 Toluene 245.5
Cyclohexane 239.3 Methanol 88.8 Triethyl mine 297.8
Source: Meissner, Chem. Eng. Prog., 45, 149-153 (1949).
Table 3.6 Structural Contributions for Estimating the Parachor
Alkyl groups
1-Methylethyl 0 (not noted above)
1-Methylpropyl N (not noted above)
1 -Methylbutyl s
2-Methylpropyl P
1-Ethylpropyl F
1,l-Dimethylethyl C1
1,l-Dimethylpropyl Br
1,2-Dimethylpropyl I
1,1,2-Trimethylpropyl Ethylenic bonds:
C6H5 Terminal
2,3-position
Special groups: 3,4-position
-coo-
-COOH Triple bond
-OH
-NH2 Ring closure:
-0- Three-membered
-NO2 Four-membered
-NO3 (nitrate) Five-membered
-CO(NH2) Six-membered
Source: Quale [a].
Estimate the diffusivity of formic acid (A) in benzene (B) at 25°C However, because formic acid is an organic acid, ??A is doubled
and infinite dilution, using the appropriate correlation of Hayduk to 187.4.
and Minhas [7]. The experimental value is 2.28 x cm2/s.
From (3-42),
SOLUTION [298'~29(205.30~5/ 187.4°.42)]
0.60.92960.23
(DAB)co = 1.55 x
Equation (3-42) applies, with T = 298 K
= 2.15 x 10-' crn21s
YA = 93.7 ~m~-~~/~/s~/~-mol
9g = 205.3 ~m~-~~/~/s'/~-mol
=
IJ.~ 0.6 cP at 25°C vg = 96 cm3/mol at 80°C which is within 6% of the the experimental value.