Page 175 - Soil and water contamination, 2nd edition
P. 175
162 Soil and Water Contamination
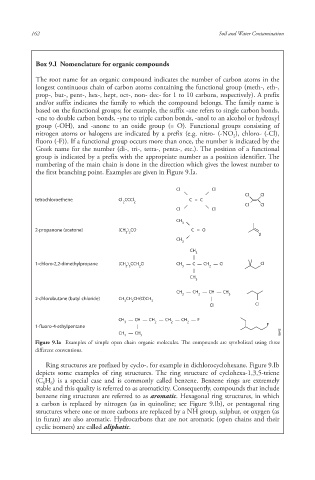
Box 9.I Nomenclature for organic compounds
The root name for an organic compound indicates the number of carbon atoms in the
longest continuous chain of carbon atoms containing the functional group (meth-, eth-,
prop-, but-, pent-, hex-, hept, oct-, non- dec- for 1 to 10 carbons, respectively). A prefix
and/or suffix indicates the family to which the compound belongs. The family name is
based on the functional groups; for example, the suffix -ane refers to single carbon bonds,
-ene to double carbon bonds, -yne to triple carbon bonds, -anol to an alcohol or hydroxyl
group (-OH), and -anone to an oxide group (= O). Functional groups consisting of
nitrogen atoms or halogens are indicated by a prefix (e.g. nitro- (-NO ), chloro- (-Cl),
2
fluoro (-F)). If a functional group occurs more than once, the number is indicated by the
Greek name for the number (di-, tri-, tetra-, penta-, etc.). The position of a functional
group is indicated by a prefix with the appropriate number as a position identifier. The
numbering of the main chain is done in the direction which gives the lowest number to
the first branching point. Examples are given in Figure 9.Ia.
Cl Cl
Cl Cl
tetrachloroethene Cl CCCl C = C
2 2
Cl Cl
Cl Cl
CH
3
2-propanone (acetone) (CH ) CO C = O
3 2
0
CH
3
CH
3
1-chloro-2,2-dimethylpropane (CH ) CCH Cl CH C CH Cl Cl
3 3 2 3 2
CH
3
CH CH CH CH
3 2 3
2-chlorobutane (butyl chloride) CH CH CH(Cl)CH
3 2 3
Cl Cl
CH CH CH CH CH F
3 2 2 2
1-fluoro-4-ethylpentane F
CH CH 6642
3 2
Figure 9.Ia Examples of simple open chain organic molecules. The compounds are symbolised using three
different conventions.
Ring structures are prefixed by cyclo-, for example in dichlorocyclohexane. Figure 9.Ib
depicts some examples of ring structures. The ring structure of cyclohexa-1,3,5-triene
(C H ) is a special case and is commonly called benzene. Benzene rings are extremely
6 6
stable and this quality is referred to as aromaticity. Consequently, compounds that include
benzene ring structures are referred to as aromatic. Hexagonal ring structures, in which
a carbon is replaced by nitrogen (as in quinoline; see Figure 9.Ib), or pentagonal ring
structures where one or more carbons are replaced by a NH group, sulphur, or oxygen (as
in furan) are also aromatic. Hydrocarbons that are not aromatic (open chains and their
cyclic isomers) are called aliphatic.
10/1/2013 6:44:39 PM
Soil and Water.indd 174 10/1/2013 6:44:39 PM
Soil and Water.indd 174