Page 60 - Strategies and Applications in Quantum Chemistry From Molecular Astrophysics to Molecular Engineer
P. 60
A COUPLED MCSCF-PERTURBATION TREATMENT OF ELECTRONIC SPECTRA 45
"chemical" (or spectroscopic, or quantum chemical... ) intuition can help in designing the
most relevant CI space as will be shown in the case of in the next section. In
particular, CAS spaces which are often used to build zeroth-order wavefunctions before
performing large-scale CI can be split into products of smaller CAS or GVB [47] spaces
without loss of accuracy: the formal completeness of the treatment may be lost, but the
computing time saving is considerable.
It is furthermore logical to use some sets of orbitals that are coherent with the zeroth-order
space used: the natural MCSCF orbitals issued from an MCSCF treatment using the space
defined previously are then attractive candidates for the perturbation.
Finally, in order to ensure an homogeneous treatment of all excited states at the variational
level, the MCSCF calculation should be averaged on the states under investigation. The
lowest eigenfunctions of the MCSCF Hamiltonian will provide the zeroth-order
wavefunctions to build the perturbation on.
As a conclusion, the calculation will be performed using a state-averaged MCSCF treatment
in a well-designed active space.
3.2. THE ACTIVE SPACE FOR
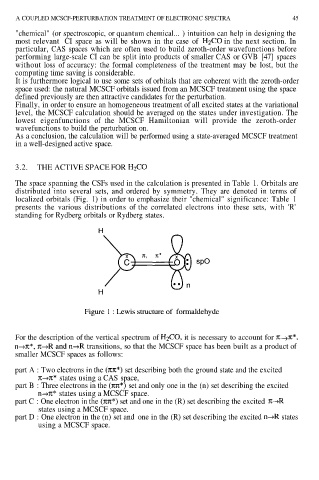
The space spanning the CSFs used in the calculation is presented in Table 1. Orbitals are
distributed into several sets, and ordered by symmetry. They are denoted in terms of
localized orbitals (Fig. 1) in order to emphasize their "chemical" significance: Table 1
presents the various distributions of the correlated electrons into these sets, with 'R'
standing for Rydberg orbitals or Rydberg states.
For the description of the vertical spectrum of it is necessary to account for
transitions, so that the MCSCF space has been built as a product of
smaller MCSCF spaces as follows:
part A : Two electrons in the set describing both the ground state and the excited
states using a CAS space,
part B : Three electrons in the set and only one in the (n) set describing the excited
states using a MCSCF space.
part C : One electron in the set and one in the (R) set describing the excited
states using a MCSCF space.
part D : One electron in the (n) set and one in the (R) set describing the excited states
using a MCSCF space.