Page 288 - Synthetic Fuels Handbook
P. 288
274 CHAPTER NINE
before transportation. Straw burning requires a specific technology. There are four basic
types of straw burners: (a) those that accept shredded, loose straw; (b) burners that use den-
sified straw products such as pellets, briquettes, or cubes, and straw logs; (c) small, square
bale burners and; (d) round bale burners. To be suitable for heat and electricity production
straw should not have a large content of moisture, preferably not more than 20 percent
as the moisture reduces the boiler efficiency. Also straw color as well as straw chemistry
should be considered before burning as it indicates the quality of the straw.
The agro-industrial residues are by-products of the postharvest processes of crops such
as cleaning, threshing, sieving, and crushing. These could be in the form of husk, dust, and
straw. Furthermore, the quantity of agricultural residues produced differs from crop to crop
and is affected by soil type and irrigation conditions. Production of agricultural residues
is directly related to the corresponding crop production and ratio between the main crop
produce and the residues, which varies from crop to crop and, at times, with the variety of
the seeds in one crop itself. Thus, for known amounts of crop production, it may be possible
to estimate the amounts of agricultural residues produced using the residue-to-crop ratio.
Most crop or agricultural residues are not found throughout the year but are available
only at the time of harvest. The amount available depends upon the harvesting time, stor-
age-related characteristics, and the storage facility.
9.2.3 Pyrolysis
Pyrolysis is a medium temperature method which produces gas, oil, and char from crops
which can then be further processed into useful fuels or feedstock (Boateng et al., 2007).
Pyrolysis is often considered to be the gasification of biomass in the absence of oxygen.
However, the chemistry of each process may differ significantly. In general, biomass does
not gasify as easily as coal, and it produces other hydrocarbon compounds in the gas mix-
ture exiting the gasifier; this is especially true when no oxygen is used. As a result, typically
an extra step must be taken to reform these hydrocarbons with a catalyst to yield a clean
syngas mixture of hydrogen, carbon monoxide, and carbon dioxide.
Fast pyrolysis is a thermal decomposition process that occurs at moderate temperatures
with a high heat transfer rate to the biomass particles and a short hot vapor residence time in
the reaction zone. Several reactor configurations have been shown to assure this condition
and to achieve yields of liquid product as high as 75 percent based on the starting dry bio-
mass weight. They include bubbling fluid beds, circulating and transported beds, cyclonic
reactors, and ablative reactors.
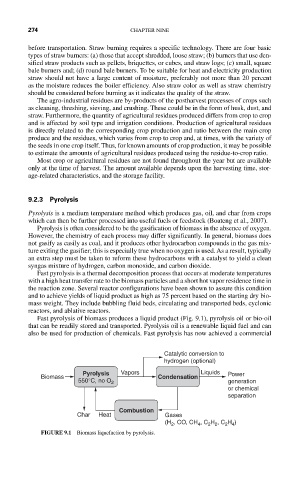
Fast pyrolysis of biomass produces a liquid product (Fig. 9.1), pyrolysis oil or bio-oil
that can be readily stored and transported. Pyrolysis oil is a renewable liquid fuel and can
also be used for production of chemicals. Fast pyrolysis has now achieved a commercial
Catalytic conversion to
hydrogen (optional)
Pyrolysis Vapors Liquids Power
Biomass Condensation
550°C, no O 2 generation
or chemical
separation
Combustion
Char Heat Gases
(H , CO, CH , C H , C H )
2 4
2 2
2
4
FIGURE 9.1 Biomass liquefaction by pyrolysis.