Page 189 - Tandem Techniques
P. 189
Page 172
2. There is an exchange of charge between the sample molecule and the reagent ion,
3. There is simple addition of the sample molecule to the reagent ion,
4. Finally there can be anion extraction,
As an example (CH +) ions, which are formed when methane is used as the reagent gas, will react with
5
a sample molecule largely by proton transfer e.g.,
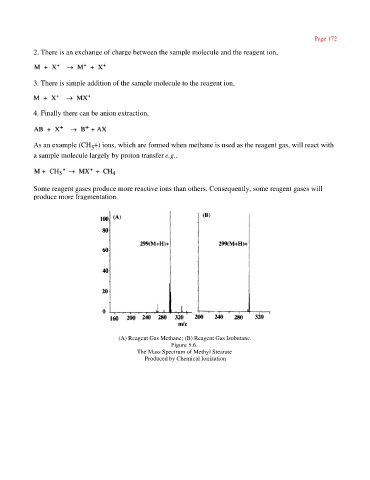
Some reagent gases produce more reactive ions than others. Consequently, some reagent gases will
produce more fragmentation.
(A) Reagent Gas Methane; (B) Reagent Gas Isobutane.
Figure 5.6.
The Mass Spectrum of Methyl Stearate
Produced by Chemical Ionization