Page 499 - Tandem Techniques
P. 499
Page 485
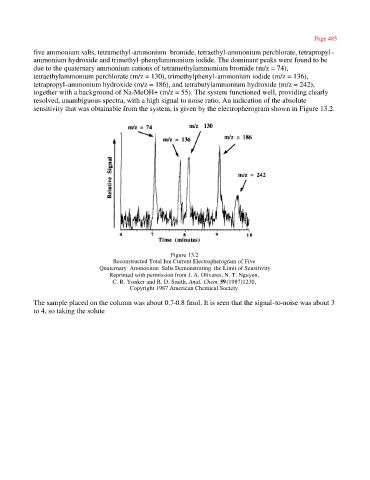
five ammonium salts, tetramethyl-ammonium bromide, tetraethyl-ammonium perchlorate, tetrapropyl-
ammonium hydroxide and trimethyl-phenylammonium iodide. The dominant peaks were found to be
due to the quaternary ammonium cations of tetramethylammonium bromide (m/z = 74),
tetraethylammonium perchlorate (m/z = 130), trimethylphenyl-ammonium iodide (m/z = 136),
tetrapropyl-ammonium hydroxide (m/z = 186), and tetrabutylammonium hydroxide (m/z = 242),
together with a background of Na-MeOH+ (m/z = 55). The system functioned well, providing clearly
resolved, unambiguous spectra, with a high signal to noise ratio. An indication of the absolute
sensitivity that was obtainable from the system, is given by the electropherogram shown in Figure 13.2.
Figure 13.2
Reconstructed Total Ion Current Electropherogram of Five
Quaternary Ammonium Salts Demonstrating the Limit of Sensitivity
Reprinted with permission from J. A. Olivares, N. T. Nguyen,
C. R. Yonker and R. D. Smith, Anal. Chem, 59(1987)1230,
Copyright 1987 American Chemical Society.
The sample placed on the column was about 0.7-0.8 fmol. It is seen that the signal-to-noise was about 3
to 4, so taking the solute