Page 196 - The Biochemistry of Inorganic Polyphosphates
P. 196
WU095/Kulaev
WU095-08
Peculiarities of polyphosphate metabolism
180 March 9, 2004 20:32 Char Count= 0
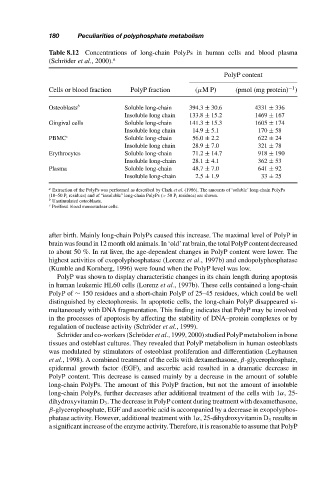
Table 8.12 Concentrations of long-chain PolyPs in human cells and blood plasma
(Schr¨oder et al., 2000). a
PolyP content
−1
Cells or blood fraction PolyP fraction (µM P) (pmol (mg protein) )
Osteoblasts b Soluble long-chain 394.3 ± 30.6 4331 ± 336
Insoluble long chain 133.8 ± 15.2 1469 ± 167
Gingival cells Soluble long-chain 141.3 ± 15.3 1605 ± 174
Insoluble long chain 14.9 ± 5.1 170 ± 58
PBMC c Soluble long-chain 56.0 ± 2.2 622 ± 24
Insoluble long chain 28.9 ± 7.0 321 ± 78
Erythrocytes Soluble long-chain 71.2 ± 14.7 918 ± 190
Insoluble long-chain 28.1 ± 4.1 362 ± 53
Plasma Soluble long-chain 48.7 ± 7.0 641 ± 92
Insoluble long-chain 2.5 ± 1.9 33 ± 25
a Extraction of the PolyPs was performed as described by Clark et al. (1986). The amounts of ‘soluble’ long-chain PolyPs
(10–50 P i residues) and of “insoluble” long-chain PolyPs (> 50 P i residues) are shown.
b Unstimulated osteoblasts.
c Periferal blood mononuclear cells.
after birth. Mainly long-chain PolyPs caused this increase. The maximal level of PolyP in
brain was found in 12 month old animals. In ‘old’ rat brain, the total PolyP content decreased
to about 50 %. In rat liver, the age-dependent changes in PolyP content were lower. The
highest activities of exopolyphosphatase (Lorenz et al., 1997b) and endopolyphosphatase
(Kumble and Kornberg, 1996) were found when the PolyP level was low.
PolyP was shown to display characteristic changes in its chain length during apoptosis
in human leukemic HL60 cells (Lorenz et al., 1997b). These cells contained a long-chain
PolyP of ∼ 150 residues and a short-chain PolyP of 25–45 residues, which could be well
distinguished by electophoresis. In apoptotic cells, the long-chain PolyP disappeared si-
multaneously with DNA fragmentation. This finding indicates that PolyP may be involved
in the processes of apoptosis by affecting the stability of DNA–protein complexes or by
regulation of nuclease activity (Schr¨oder et al., 1999).
Schr¨oder and co-workers (Schr¨oder et al., 1999, 2000) studied PolyP metabolism in bone
tissues and osteblast cultures. They revealed that PolyP metabolism in human osteoblasts
was modulated by stimulators of osteoblast proliferation and differentiation (Leyhausen
et al., 1998). A combined treatment of the cells with dexamethasone, β-glycerophosphate,
epidermal growth factor (EGF), and ascorbic acid resulted in a dramatic decrease in
PolyP content. This decrease is caused mainly by a decrease in the amount of soluble
long-chain PolyPs. The amount of this PolyP fraction, but not the amount of insoluble
long-chain PolyPs, further decreases after additional treatment of the cells with 1α, 25-
dihydroxyvitamin D 3 . The decrease in PolyP content during treatment with dexamethasone,
β-glycerophosphate, EGF and ascorbic acid is accompanied by a decrease in exopolyphos-
phatase activity. However, additional treatment with 1α, 25-dihydroxyvitamin D 3 results in
a significant increase of the enzyme activity. Therefore, it is reasonable to assume that PolyP