Page 194 - Thermal Hydraulics Aspects of Liquid Metal Cooled Nuclear Reactors
P. 194
System thermal hydraulics for liquid metals 165
when there is a relative acceleration between the two phases. This contribute is impor-
tant only when the velocity is close to the sound speed in the fluid:
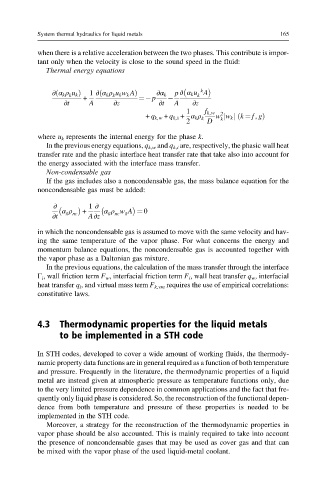
Thermal energy equations
k
∂ α k ρ u k Þ + 1 ∂ α k ρ u k w k AÞ ¼ p ∂α k p ∂ α k u A
ð
ð
k
k
k
∂t A ∂z ∂t A ∂z
1 f k,w
2
+ q k,w + q k,i + α k ρ k w w k j k ¼ f, gð Þ
j
2 D k
where u k represents the internal energy for the phase k.
In the previous energy equations, q k,w and q k,i are, respectively, the phasic wall heat
transfer rate and the phasic interface heat transfer rate that take also into account for
the energy associated with the interface mass transfer.
Non-condensable gas
If the gas includes also a noncondensable gas, the mass balance equation for the
noncondensable gas must be added:
∂ 1 ∂
α g ρ + α g ρ w g A ¼ 0
∂t nc A∂z nc
in which the noncondensable gas is assumed to move with the same velocity and hav-
ing the same temperature of the vapor phase. For what concerns the energy and
momentum balance equations, the noncondensable gas is accounted together with
the vapor phase as a Daltonian gas mixture.
In the previous equations, the calculation of the mass transfer through the interface
Γ i , wall friction term F w , interfacial friction term F i , wall heat transfer q w , interfacial
heat transfer q i , and virtual mass term F k,vm requires the use of empirical correlations:
constitutive laws.
4.3 Thermodynamic properties for the liquid metals
to be implemented in a STH code
In STH codes, developed to cover a wide amount of working fluids, the thermody-
namic property data functions are in general required as a function of both temperature
and pressure. Frequently in the literature, the thermodynamic properties of a liquid
metal are instead given at atmospheric pressure as temperature functions only, due
to the very limited pressure dependence in common applications and the fact that fre-
quently only liquid phase is considered. So, the reconstruction of the functional depen-
dence from both temperature and pressure of these properties is needed to be
implemented in the STH code.
Moreover, a strategy for the reconstruction of the thermodynamic properties in
vapor phase should be also accounted. This is mainly required to take into account
the presence of noncondensable gases that may be used as cover gas and that can
be mixed with the vapor phase of the used liquid-metal coolant.