Page 45 - Thermodynamics of Biochemical Reactions
P. 45
3.2 Changes in Thermodynamic Properties in Chemical Reactions 39
G/kJ mol
21 F
I . . . / . ' S/mol
0.2 0.4 0.6 0.8 1
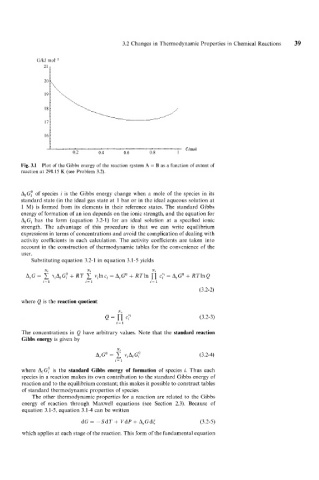
Fig. 3.1 Plot of the Gibbs energy of the reaction system A = B as a function of extent of
reaction at 298.15 K (see Problem 3.2).
A,Gp of species i is the Gibbs energy change when a mole of the species in its
standard state (in the ideal gas state at 1 bar or in the ideal aqueous solution at
1 M) is formed from its elements in their reference states. The standard Gibbs
energy of formation of an ion depends on the ionic strength, and the equation for
AfGi has the form (equation 3.2-1) for an ideal solution at a specified ionic
strength. The advantage of this procedure is that we can write equilibrium
expressions in terms of concentrations and avoid the complication of dealing with
activity coefficients in each calculation. The activity coefficients are taken into
account in the construction of thermodynamic tables for the convenience of the
user.
Substituting equation 3.2-1 in equation 3.1-5 yields
Ns N, N,
A,G = 1 viA,GY + RT vilnci = A,Go + RTln n cy' = A,Go + RTlnQ
i=l i= 1 i=l
(3.2-2)
where Q is the reaction quotient:
(3.2-3)
The concentrations in Q have arbitrary values. Note that the standard reaction
Gibbs energy is given by
(3.2-4)
where A,Gy is the standard Gibbs energy of formation of species i. Thus each
species in a reaction makes its own contribution to the standard Gibbs energy of
reaction and to the equilibrium constant; this makes it possible to construct tables
of standard thermodynamic properties of species.
The other thermodynamic properties for a reaction are related to the Gibbs
energy of reaction through Maxwell equations (see Section 2.3). Because of
equation 3.1-5, equation 3.1-4 can be written
dG = -SdT + VdP + A,,Gd< (3.2-5)
which applies at each stage of the reaction. This form of the fundamental equation