Page 60 - Thermodynamics of Biochemical Reactions
P. 60
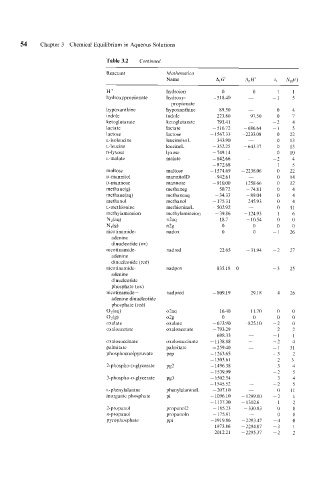
54 Chapter 3 Chemical Equilibrium in Aqueous Solutions
Table 3.2 Continued
Reactant Mathemutica
Name A, G" A, H" zi Ndi)
H+ hydroion 0 0 1 1
hydroxypropionate hydroxy- -518.40 ~ -1 5
propionate
hypoxanthine hypoxan thine 89.50 ~ 0 4
indole indole 223.80 97 50 0 7
ketoglutarate ketoglutarate - 793.41 ~ -2 4
lactate lactate -516.72 - 686 64 -1 5
lactose lactose - 1567.33 - 2233 08 0 22
L-isoleucine leucineisoL - 343.90 - 0 13
L-leucine leucineL - 352.25 - 643 37 0 13
D - 1 y x 0 s e lyxose -749.14 ~ 0 10
L-malate malate - 842.66 -2 4
- 872.68 ~ -1 5
maltose maltose - 1574.69 - 2238 06 0 22
D-mannitol mannitolD - 942.6 1 ~ 0 14
D-mannose mannose -910.00 - 1258 66 0 12
methane(g) methaneg - 50.72 -7481 0 4
methane(aq) methaneaq - 34.33 -89 04 0 4
methanol methanol - 175.31 - 245 93 0 4
L-methionine methionineL - 502.92 - 0 I1
methy lamineion methylamineion - 39.86 - 124 93 1 6
N,(4 n2aq 18.7 - 10 54 0 0
NJg) n2g 0 0 0 0
nico tinamide- nadox 0 0 -1 26
adenine
dinucleotide (ox)
nicotinamide- nadred 22.65 -71 94 -2 27
adenine
dinucleotide (red)
nico tinamide- nadpox -835.18 0 -3 25
adenine
dinucleotide
phosphate (ox)
nicotinamide - nadpred - 809.19 -29 18 -4 26
adenine dinucleotide
phosphate (red)
OAaq) o2aq 16.40 -11 70 0 0
Oh) o2g 0 0 0 0
oxalate oxalate - 673.90 -825 10 -2 0
oxaloacetate oxaloacetate - 793.29 ~ -2 2
- 698.33 ~ -1 I
oxalosuccinate oxalosuccinate - 1135.88 -2 4
palmitate palmitate - 259.40 ~ -I 31
phosphoenolpyruvate Pep - 1263.65 ~ -3 2
- 1303.61 ~ -2 3
- 1496.38 ~ -3 4
- 1539.99 -2 5
- 1502.54 - -3 4
- 1545.52 ~ -2 5
L-phen ylalanine phenylalanineL - 207.10 ~ 0 11
inorganic phosphate Pi - 1096.10 - 1299 00 -2 1
- 1137.30 -13026 -I 2
2-propanol propano12 - 185.23 - 330 83 0 8
n-propanol propanoln - 175.81 - 0 8
pyrophosphate PPi - 191 9.86 - 2293 47 -4 0
- 1973.86 - 2294 87 -3 I
-2012.21 - 2295 37 -2 2