Page 101 - Vogel's TEXTBOOK OF QUANTITATIVE CHEMICAL ANALYSIS
P. 101
THE ANALYTICAL BALANCE 3.2
measurement of mass in such operations, an analytical balance is employed;
the operation is referred to as weighing, and invariably reference is made to the
weight of the object or material which is weighed.
The weight of an object is the force of attraction due to gravity which is
exerted upon the object:
where w is the weight of the object, m its mass, and g is the acceleration due
to gravity. Since the attraction due to gravity varies over the earth's surface
with altitude and also with latitude, the weight of the object is variable, whereas
its mass is constant. It has however become the custom to employ the term
'weight' synonymously with mass, and it is in this sense that 'weight' is employed
in quantitative analysis.
The analytical balance is thus one of the most important tools of the analytical
chemist, and it is one which of recent years has undergone radical changes.
These changes have been prompted by the desire to produce an instrument
which is more robust, less dependent upon the experience of the operator, less
susceptible to the environment, and above aii, one which will hasten the weighing
operation. In meeting these requirements, the design of the balance has been
fundamentally altered, and the conventional free-swinging, equal-am, two-pan
chemical balance together with its box of weights is now an uncommon sight.
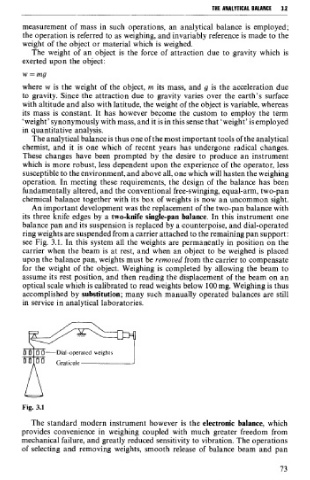
An important development was the replacement of the two-pan balance with
its three knife edges by a two-knife single-pan balance. In this instrument one
balance pan and its suspension is replaced by a counterpoise, and dial-operated
ring weights are suspended from a carrier attached to the remaining pan support:
see Fig. 3.1. In this system al1 the weights are permanently in position on the
carrier when the beam is at rest, and when an object to be weighed is placed
upon the balance pan, weights must be remooed from the carrier to compensate
for the weight of the object. Weighing is completed by allowing the beam to
assume its rest position, and then reading the displacement of the beam on an
optical scale which is calibrated to read weights below 100 mg. Weighing is thus
accomplished by substitution; many such manually operated balances are still
in service in analytical laboratories.
Fig. 3.1
The standard modern instrument however is the electronic balance, which
provides convenience in weighing coupled with much greater freedom from
mechanical failure, and greatly reduced sensitivity to vibration. The operations
of selecting and removing weights, smooth release of balance beam and pan