Page 118 - Vogel's TEXTBOOK OF QUANTITATIVE CHEMICAL ANALYSIS
P. 118
3 COMMON APPARATUS AND BASIC TECHNIQUES
Grade 2 water to reverse osmosis or de-ionisation, followed by filtration
through a membrane filter of pore size 0.2 pm to remove particulate matter.
Alternatively, Grade 2 water may be redistilled in an apparatus constructed
from fused silica.
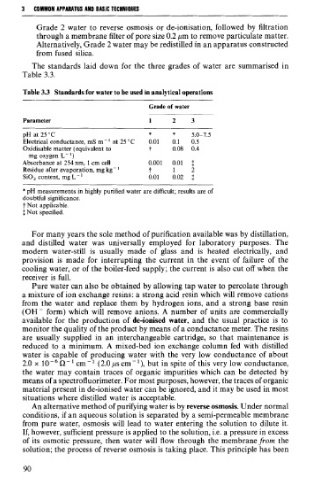
The standards laid down for the three grades of water are summarised in
Table 3.3.
Table 3.3 Standards for water to be used in analytical operations
Grade of water
Parameter 1 2 3
pH at 25 "C * * 5.0-7.5
Electrical conductance, mS m-' at 25°C 0.01 0.1 0.5
Oxidisable matter (equivalent to ? 0.08 0.4
mg oxygen L- ')
Absorbance at 254 nm, 1 cm cell 0.001 0.01 $
Residue after evaporation, mg kg - ' t 1 2
SiO, content, mg L- ' 0.01 0.02 $
*pH measurements in highly purified water are difficult; results are of
doubtful significance.
t Not applicable.
$ Not specified.
For many years the sole method of purification available was by distillation,
and distilled water was universally employed for laboratory purposes. The
modern water-still is usually made of glass and is heated electrically, and
provision is made for interrupting the current in the event of failure of the
cooling water, or of the boiler-feed supply; the current is also cut off when the
receiver is full.
Pure water can also be obtained by allowing tap water to percolate through
a mixture of ion exchange resins: a strong acid resin which will remove cations
from the water and replace them by hydrogen ions, and a strong base resin
(OH - form) which will remove anions. A number of units are commercially
available for the production of de-ionised water, and the usual practice is to
monitor the quality of the product by means of a conductance meter. The resins
are usually supplied in an interchangeable cartridge, so that maintenance is
reduced to a minimum. A mixed-bed ion exchange column fed with distilled
water is capable of producing water with the very low conductance of about
'
2.0 x 0- cm - ' (2.0 ps cm - '), but in spite of this very low conductance,
the water may contain traces of organic impurities which can be detected by
means of a spectrofluorimeter. For most purposes, however, the traces of organic
material present in de-ionised water can be ignored, and it may be used in most
situations where distilled water is acceptable.
An alternative method of purifying water is by reverse osmosis. Under normal
conditions, if an aqueous solution is separated by a semi-permeable membrane
from pure water, osmosis wiil lead to water entering the solution to dilute it.
If, however, sufficient pressure is applied to the solution, i.e. a pressure in excess
of its osmotic pressure, then water will flow through the membrane from the
solution; the process of reverse osmosis is taking place. This principle has been