Page 214 - Vogel's TEXTBOOK OF QUANTITATIVE CHEMICAL ANALYSIS
P. 214
CHAPTER 7
ION EXCHANGE
7.1 GENERAL DISCUSSION
The term ion exchange is generally understood to mean the exchange of ions
of like sign between a solution and a solid highly insoluble body in contact with
it. The solid (ion exchanger) must, of course, contain ions of its own, and for
the exchange to proceed sufficiently rapidly and extensively to be of practical
value, the solid must have an open, permeable molecular structure so that ions
and solvent molecules can move freely in and out. Many substances, both natural
(e.g. certain clay minerals) and artificial, have ion exchanging properties, but
for analytical work synthetic organic ion exchangers are chiefly of interest,
although some inorganic materials, e.g. zirconyl phosphate and ammonium
12-molybdophosphate, also possess useful ion exchange capabilities and have
specialised application^.'^ Al1 ion exchangers of value in analysis have several
properties in common, they are almost insoluble in water and in organic solvents,
and they contain active or counter-ions that will exchange reversibly with other
ions in a surrounding solution without any appreciable physical change
occurring in the material. The ion exchanger is of complex nature and is, in
fact, polymeric. The polymer carries an electric charge that is exactly neutralised
by the charges on the counter-ions. These active ions are cations in a cation
exchanger and anions in an anion exchanger. Thus a cation exchanger consists
of a polymeric anion and active cations, while an anion exchanger is a polymeric
cation with active anions.
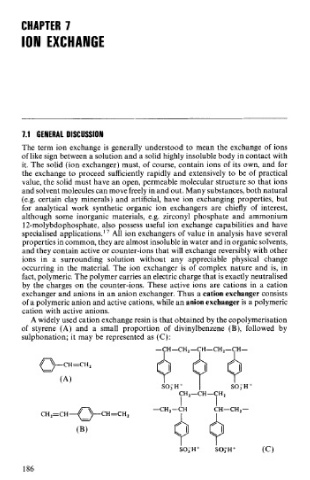
A widely used cation exchange resin is that obtained by the copolymerisation
of styrene (A) and a small proportion of divinylbenzene (B), followed by
sulphonation; it may be represented as (C):