Page 291 - Vogel's TEXTBOOK OF QUANTITATIVE CHEMICAL ANALYSIS
P. 291
NEUTRALISATION INOICATORS 10.7
chief characteristic of these indicators is that the change from a predominantly
'acid' colour to a predominantly 'alkaline' colour is not sudden and abrupt,
but takes place within a small interval of pH (usually about two pH units)
termed the colour-change interval of the indicator. The position of the colour-
change interval in the pH scale varies widely with different indicators. For most
acid-base titrations it is possible to select an indicator which exhibits a distinct
colour change at a pH close to that corresponding to the equivalence
point.
The first useful theory of indicator action was suggested by W. 0stwald3 based
upon the concept that indicators in general use are very weak organic acids or
bases.
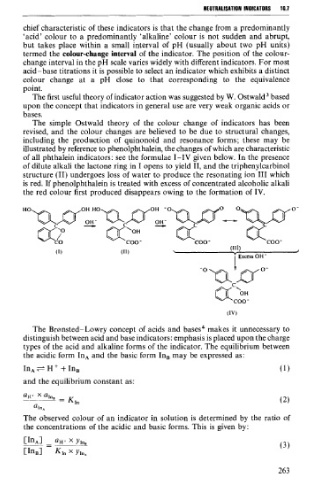
The simple Ostwald theory of the colour change of indicators has been
revised, and the colour changes are believed to be due to structural changes,
including the production of quinonoid and resonance forms; these may be
illustrated by reference to phenolphthalein, the changes of which are characteristic
of al1 phthalein indicators: see the formulae 1-IV given below. In the presence
of dilute alkali the lactone ring in 1 opens to yield II, and the triphenylcarbinol
structure (II) undergoes loss of water to produce the resonating ion III which
is red. If phenolphthalein is treated with excess of concentrated alcoholic alkali
the red colour first produced disappears owing to the formation of IV.
The Brunsted-Lowry concept of acids and bases4 makes it unnecessary to
distinguish between acid and base indicators: emphasis is placed upon the charge
types of the acid and alkaline forms of the indicator. The equilibrium between
the acidic form In, and the basic form In, may be expressed as:
In, e H+ +In, (1)
and the equilibrium constant as:
a~~ X alnB -
- KI, (2)
a,,*
The observed colour of an indicator in solution is determined by the ratio of
the concentrations of the acidic and basic forms. This is given by: