Page 274 - Water and wastewater engineering
P. 274
COAGULATION AND FLOCCULATION 6-51
6-4. Calculate the “approximate” alkalinity (in mg/L as CaCO 3 ) of a water containing
15 mg/L of bicarbonate ion and 120 mg/L of carbonate ion.
6-5. A jar test has shown that the optimum dose of ferric chloride consumes all of the
alkalinity. If the amount of ferric chloride that is in excess is 10 mg/L, how much
lime (Ca(OH) 2 ), in mg/L must be added to neutralize the acid formed?
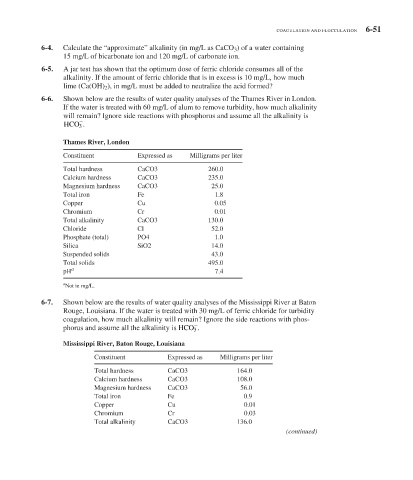
6-6. Shown below are the results of water quality analyses of the Thames River in London.
If the water is treated with 60 mg/L of alum to remove turbidity, how much alkalinity
will remain? Ignore side reactions with phosphorus and assume all the alkalinity is
HCO 3 .
Thames River, London
Constituent Expressed as Milligrams per liter
Total hardness CaCO3 260.0
Calcium hardness CaCO3 235.0
Magnesium hardness CaCO3 25.0
Total iron Fe 1.8
Copper Cu 0.05
Chromium Cr 0.01
Total alkalinity CaCO3 130.0
Chloride Cl 52.0
Phosphate (total) PO4 1.0
Silica SiO2 14.0
Suspended solids 43.0
Total solids 495.0
pH a 7.4
a
Not in mg/L.
6-7. Shown below are the results of water quality analyses of the Mississippi River at Baton
Rouge, Louisiana. If the water is treated with 30 mg/L of ferric chloride for turbidity
coagulation, how much alkalinity will remain? Ignore the side reactions with phos-
phorus and assume all the alkalinity is HCO 3 .
Mississippi River, Baton Rouge, Louisiana
Constituent Expressed as Milligrams per liter
Total hardness CaCO3 164.0
Calcium hardness CaCO3 108.0
Magnesium hardness CaCO3 56.0
Total iron Fe 0.9
Copper Cu 0.01
Chromium Cr 0.03
Total alkalinity CaCO3 136.0
(continued)