Page 241 - Characterization and Properties of Petroleum Fractions - M.R. Riazi
P. 241
T1: IML
QC: IML/FFX
P2: IML/FFX
P1: IML/FFX
August 16, 2007
17:42
AT029-Manual-v7.cls
AT029-05
AT029-Manual
5. PVT RELATIONS AND EQUATIONS OF STATE 221
1.4
0.6
Gases
1.2
4
1.0
2
T r = 0.6
0.8 1.5
0.8
(0) Two-Phase
Z 1.0
--`,```,`,``````,`,````,```,,-`-`,,`,,`,`,,`---
0.6 Region Gases
0.4
Compressed
0.2 Liquids
0.8
T r < 1
0.0
0.01 0.1 1 10
(a)
Reduced Pressure, Pr
0.4
2
Gases
0.2 4
1.5
0.0
0.8 1.0
T r = 0.6 Vapor
-0.2
(1)
Z
Compressed 0.8
Liquids
-0.4
Tr < 1 0.6
-0.6
-0.8
0.01 0.1 1 10
(b) Reduced Pressure, Pr
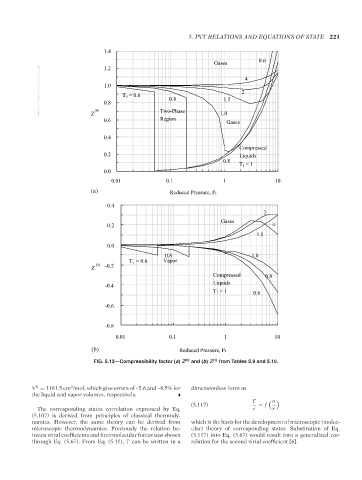
FIG. 5.13—Compressibility factor (a) Z (0) and (b) Z (1) from Tables 5.9 and 5.10.
V
3
V = 1161.5cm /mol, which give errors of –5.6 and –4.5% for dimensionless form as
the liquid and vapor volumes, respectively.
σ
(5.117) = f
The corresponding states correlation expressed by Eq. ε r
(5.107) is derived from principles of classical thermody-
namics. However, the same theory can be derived from which is the basis for the development of microscopic (molec-
microscopic thermodynamics. Previously the relation be- ular) theory of corresponding states. Substitution of Eq.
tween virial coefficients and intermolecular forces was shown (5.117) into Eq. (5.67) would result into a generalized cor-
through Eq. (5.67). From Eq. (5.11), can be written in a relation for the second virial coefficient [6].
Copyright ASTM International
Provided by IHS Markit under license with ASTM Licensee=International Dealers Demo/2222333001, User=Anggiansah, Erick
No reproduction or networking permitted without license from IHS Not for Resale, 08/26/2021 21:56:35 MDT