Page 58 - Mechanical Behavior of Materials
P. 58
Section 2.5 Inelastic Deformation 59
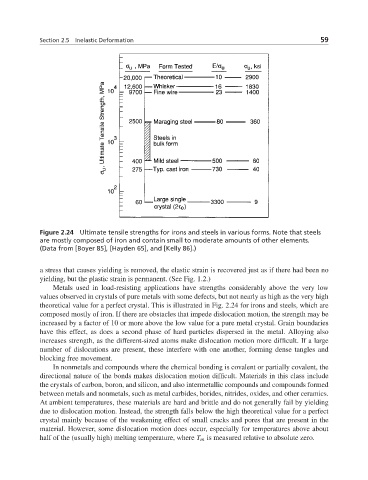
Figure 2.24 Ultimate tensile strengths for irons and steels in various forms. Note that steels
are mostly composed of iron and contain small to moderate amounts of other elements.
(Data from [Boyer 85], [Hayden 65], and [Kelly 86].)
a stress that causes yielding is removed, the elastic strain is recovered just as if there had been no
yielding, but the plastic strain is permanent. (See Fig. 1.2.)
Metals used in load-resisting applications have strengths considerably above the very low
values observed in crystals of pure metals with some defects, but not nearly as high as the very high
theoretical value for a perfect crystal. This is illustrated in Fig. 2.24 for irons and steels, which are
composed mostly of iron. If there are obstacles that impede dislocation motion, the strength may be
increased by a factor of 10 or more above the low value for a pure metal crystal. Grain boundaries
have this effect, as does a second phase of hard particles dispersed in the metal. Alloying also
increases strength, as the different-sized atoms make dislocation motion more difficult. If a large
number of dislocations are present, these interfere with one another, forming dense tangles and
blocking free movement.
In nonmetals and compounds where the chemical bonding is covalent or partially covalent, the
directional nature of the bonds makes dislocation motion difficult. Materials in this class include
the crystals of carbon, boron, and silicon, and also intermetallic compounds and compounds formed
between metals and nonmetals, such as metal carbides, borides, nitrides, oxides, and other ceramics.
At ambient temperatures, these materials are hard and brittle and do not generally fail by yielding
due to dislocation motion. Instead, the strength falls below the high theoretical value for a perfect
crystal mainly because of the weakening effect of small cracks and pores that are present in the
material. However, some dislocation motion does occur, especially for temperatures above about
half of the (usually high) melting temperature, where T m is measured relative to absolute zero.