Page 200 - Algae Anatomy, Biochemistry, and Biotechnology
P. 200
Working with Light 183
In the case of solar radiation, scattering is due to its interaction with gas molecules and sus-
pended particles found in the atmosphere. Scattering reduces the amount of incoming radiation
reaching the Earth’s surface because significant proportion of solar radiation is redirected back
to space. The amount of scattering that takes place is dependent on two factors: wavelength of
the incoming radiation and size of the scattering particle or gas molecule. For small particles
compared to the visible radiation, Rayleigh’s scattering theory holds. It states that the intensity
of scattered waves roughly in the same direction of the incoming radiation is inversely proportional
to the fourth power of the wavelength. In the Earth’s atmosphere, the presence of a large number of
small particles compared to the visible radiation (with a size of about 0.5 mm) results such that the
shorter wavelengths of the visible range are more intensely diffused. This factor causes our sky to
look blue because this color corresponds to those wavelengths. When the scattering particles are
very much larger than the wavelength, then the intensity of scattered waves roughly in the same
direction of the incoming radiation become independent of wavelength and for this reason, the
clouds, made of large raindrops, are white. If scattering does not occur in our atmosphere the
daylight sky would be black.
ABSORPTION:LAMBERT–BEER LAW
Some molecules have the ability to absorb incoming light. Absorption is defined as a process in
which light is retained by a molecule. In this way, the free energy of the photon absorbed by the
molecule can be used to carry out work, emitted as fluorescence or dissipated as heat.
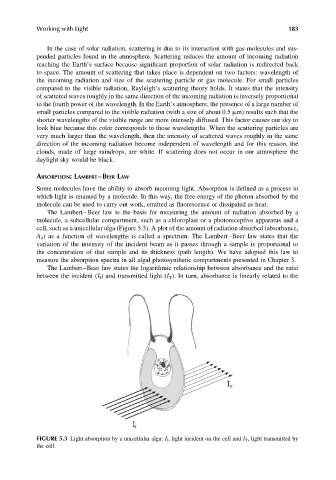
The Lambert–Beer law is the basis for measuring the amount of radiation absorbed by a
molecule, a subcellular compartment, such as a chloroplast or a photoreceptive apparatus and a
cell, such as a unicellular alga (Figure 5.3). A plot of the amount of radiation absorbed (absorbance,
A l ) as a function of wavelengths is called a spectrum. The Lambert–Beer law states that the
variation of the intensity of the incident beam as it passes through a sample is proportional to
the concentration of that sample and its thickness (path length). We have adopted this law to
measure the absorption spectra in all algal photosynthetic compartments presented in Chapter 3.
The Lambert–Beer law states the logarithmic relationship between absorbance and the ratio
between the incident (I I ) and transmitted light (I T ). In turn, absorbance is linearly related to the
FIGURE 5.3 Light absorption by a unicellular alga: I I , light incident on the cell and I T , light transmitted by
the cell.