Page 262 - Algae Anatomy, Biochemistry, and Biotechnology
P. 262
Algal Culturing 245
tech,” and can be used also in remote locations, other are “high-tech,” and require an expensive
instrument.
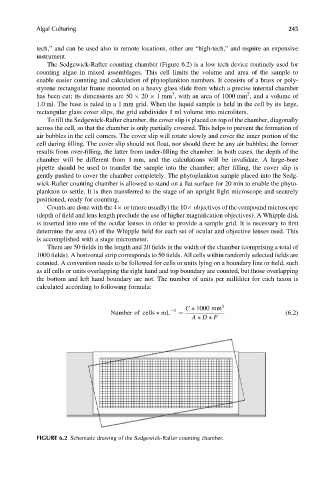
The Sedgewick-Rafter counting chamber (Figure 6.2) is a low tech device routinely used for
counting algae in mixed assemblages. This cell limits the volume and area of the sample to
enable easier counting and calculation of phytoplankton numbers. It consists of a brass or poly-
styrene rectangular frame mounted on a heavy glass slide from which a precise internal chamber
3
2
has been cut; its dimensions are 50 20 1mm , with an area of 1000 mm , and a volume of
1.0 ml. The base is ruled in a 1 mm grid. When the liquid sample is held in the cell by its large,
rectangular glass cover slips, the grid subdivides 1 ml volume into microliters.
To fill the Sedgewick-Rafter chamber, the cover slip is placed on top of the chamber, diagonally
across the cell, so that the chamber is only partially covered. This helps to prevent the formation of
air bubbles in the cell corners. The cover slip will rotate slowly and cover the inner portion of the
cell during filling. The cover slip should not float, nor should there be any air bubbles; the former
results from over-filling, the latter from under-filling the chamber. In both cases, the depth of the
chamber will be different from 1 mm, and the calculations will be invalidate. A large-bore
pipette should be used to transfer the sample into the chamber; after filling, the cover slip is
gently pushed to cover the chamber completely. The phytoplankton sample placed into the Sedg-
wick-Rafter counting chamber is allowed to stand on a flat surface for 20 min to enable the phyto-
plankton to settle. It is then transferred to the stage of an upright light microscope and securely
positioned, ready for counting.
Counts are done with the 4 or (more usually) the 10 objectives of the compound microscope
(depth of field and lens length preclude the use of higher magnification objectives). A Whipple disk
is inserted into one of the ocular lenses in order to provide a sample grid. It is necessary to first
determine the area (A) of the Whipple field for each set of ocular and objective lenses used. This
is accomplished with a stage micrometer.
There are 50 fields in the length and 20 fields in the width of the chamber (comprising a total of
1000 fields). A horizontal strip corresponds to 50 fields. All cells within randomly selected fields are
counted. A convention needs to be followed for cells or units lying on a boundary line or field, such
as all cells or units overlapping the right hand and top boundary are counted, but those overlapping
the bottom and left hand boundary are not. The number of units per milliliter for each taxon is
calculated according to following formula:
C 1000 mm 3
1
Number of cells mL ¼ (6:2)
A D F
FIGURE 6.2 Schematic drawing of the Sedgewick-Rafter counting chamber.