Page 267 - Materials Chemistry, Second Edition
P. 267
4.5 Impact Categories, Impact Indicators and Characterisation Factors 251
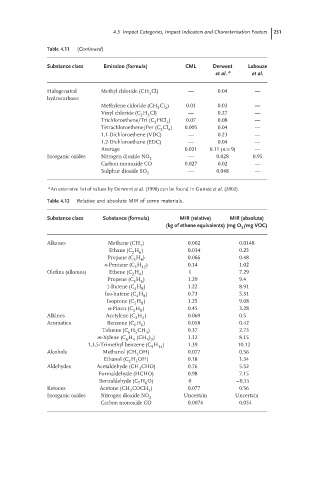
Table 4.11 (Continued)
Substance class Emission (formula) CML Derwent Labouze
et al. a et al.
Halogenated Methyl chloride (CH Cl) — 0.04 —
3
hydrocarbons
Methylene chloride (CH Cl ) 0.01 0.03 —
2
2
Vinyl chloride (C H Cl) — 0.27 —
2 3
Trichloroethene/Tri (C HCl ) 0.07 0.08 —
3
2
Tetrachloroethene/Per (C Cl ) 0.005 0.04 —
2
4
1,1-Dichloroethene (VDC) — 0.23 —
1,2-Dichloroethane (EDC) — 0.04 —
Average 0.021 0.11 (n = 9) —
Inorganic oxides Nitrogen dioxide NO 2 — 0.028 0.95
Carbon monoxide CO 0.027 0.02 —
Sulphur dioxide SO 2 — 0.048 —
a An extensive list of values by Derwent et al. (1998) can be found in Guin´ ee et al. (2002).
Table 4.12 Relative and absolute MIR of some materials.
Substance class Substance (formula) MIR (relative) MIR (absolute)
(kg of ethene equivalents) (mg O /mg VOC)
3
Alkanes Methane (CH ) 0.002 0.0148
4
Ethane (C H ) 0.034 0.25
2 6
Propane (C H ) 0.066 0.48
3 8
n-Pentane (C H ) 0.14 1.02
5
12
Olefins (alkenes) Ethene (C H ) 1 7.29
2 4
Propene (C H ) 1.29 9.4
3
6
1-Butene (C H ) 1.22 8.91
4 8
Iso-butene (C H ) 0.73 5.31
4
8
Isoprene (C H ) 1.25 9.08
5 8
α-Pinen (C H ) 0.45 3.28
5
8
Alkines Acetylene (C H ) 0.069 0.5
2 2
Aromatics Benzene (C H ) 0.058 0.42
6
6
Toluene (C H CH ) 0.37 2.73
6 5 3
m-Xylene (C H (CH ) ) 1.12 8.15
3 2
6
4
1,3,5-Trimethyl benzene (C H ) 1.39 10.12
12
9
Alcohols Methanol (CH OH) 0.077 0.56
3
Ethanol (C H OH) 0.18 1.34
2
5
Aldehydes Acetaldehyde (CH CHO) 0.76 5.52
3
Formaldehyde (HCHO) 0.98 7.15
Benzaldehyde (C H O) 0 −0.55
7 6
Ketones Acetone (CH COCH ) 0.077 0.56
3
3
Inorganic oxides Nitrogen dioxide NO Uncertain Uncertain
2
Carbon monoxide CO 0.0074 0.054