Page 176 - Materials Chemistry, Second Edition
P. 176
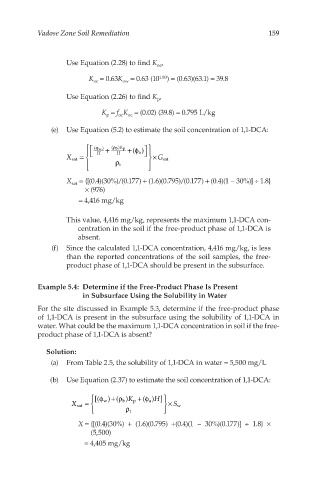
Vadose Zone Soil Remediation 159
Use Equation (2.28) to find K ,
oc
K = 0.63K = 0.63 (10 1.80 ) = (0.63)(63.1) = 39.8
ow
oc
Use Equation (2.26) to find K ,
p
K = f K = (0.02) (39.8) = 0.795 L/kg
oc
p
oc
(e) Use Equation (5.2) to estimate the soil concentration of 1,1-DCA:
( w ) + ( b ) K p + ()
ρ
ϕ
a φ
×
X sat = H H G sat
t ρ
X = {[(0.4)(30%)/(0.177) + (1.6)(0.795)/(0.177) + (0.4)(1 − 30%)] ÷ 1.8}
sat
× (976)
= 4,416 mg/kg
This value, 4,416 mg/kg, represents the maximum 1,1-DCA con-
centration in the soil if the free-product phase of 1,1-DCA is
absent.
(f) Since the calculated 1,1-DCA concentration, 4,416 mg/kg, is less
than the reported concentrations of the soil samples, the free-
product phase of 1,1-DCA should be present in the subsurface.
Example 5.4: Determine if the Free-Product Phase Is Present
in Subsurface Using the Solubility in Water
For the site discussed in Example 5.3, determine if the free-product phase
of 1,1-DCA is present in the subsurface using the solubility of 1,1-DCA in
water. What could be the maximum 1,1-DCA concentration in soil if the free-
product phase of 1,1-DCA is absent?
Solution:
(a) From Table 2.5, the solubility of 1,1-DCA in water = 5,500 mg/L
(b) Use Equation (2.37) to estimate the soil concentration of 1,1-DCA:
[( w )( )+ ρ K p + ( )]φ H
φ
X sat = b a × S w
t ρ
X = {[(0.4)(30%) + (1.6)(0.795) +(0.4)(1 − 30%)(0.177)] ÷ 1.8} ×
(5,500)
= 4,405 mg/kg