Page 288 - Materials Chemistry, Second Edition
P. 288
VOC-Laden Air Treatment 271
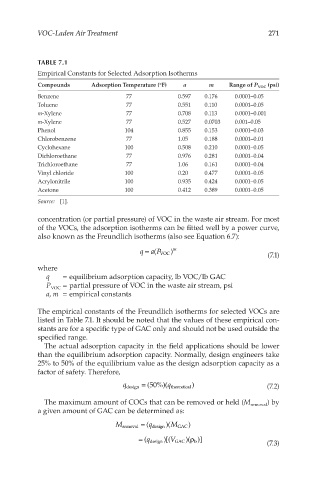
TABLE 7.1
Empirical Constants for Selected Adsorption Isotherms
Compounds Adsorption Temperature (°F) a m Range of P VOC (psi)
Benzene 77 0.597 0.176 0.0001–0.05
Toluene 77 0.551 0.110 0.0001–0.05
m-Xylene 77 0.708 0.113 0.0001–0.001
m-Xylene 77 0.527 0.0703 0.001–0.05
Phenol 104 0.855 0.153 0.0001–0.03
Chlorobenzene 77 1.05 0.188 0.0001–0.01
Cyclohexane 100 0.508 0.210 0.0001–0.05
Dichloroethane 77 0.976 0.281 0.0001–0.04
Trichloroethane 77 1.06 0.161 0.0001–0.04
Vinyl chloride 100 0.20 0.477 0.0001–0.05
Acrylonitrile 100 0.935 0.424 0.0001–0.05
Acetone 100 0.412 0.389 0.0001–0.05
Source: [1].
concentration (or partial pressure) of VOC in the waste air stream. For most
of the VOCs, the adsorption isotherms can be fitted well by a power curve,
also known as the Freundlich isotherms (also see Equation 6.7):
q = ) m
a P( VOC (7.1)
where
q = equilibrium adsorption capacity, lb VOC/lb GAC
P VOC = partial pressure of VOC in the waste air stream, psi
a, m = empirical constants
The empirical constants of the Freundlich isotherms for selected VOCs are
listed in Table 7.1. It should be noted that the values of these empirical con-
stants are for a specific type of GAC only and should not be used outside the
specified range.
The actual adsorption capacity in the field applications should be lower
than the equilibrium adsorption capacity. Normally, design engineers take
25% to 50% of the equilibrium value as the design adsorption capacity as a
factor of safety. Therefore,
q design = (50%)( ) (7.2)
q theoretical
The maximum amount of COCs that can be removed or held (M removal ) by
a given amount of GAC can be determined as:
M removal = q ( design )( M GAC )
= )[( V GAC ρ )]
q ( design )( b (7.3)