Page 362 -
P. 362
12.21
ION EXCHANGE APPLICATIONS IN WATER TREATMENT
22
I
0% SULFATES
20
18
16
14
Nitrate Selective ...........
12
10
I I F I I I I I I I I I I I I I
12
25% SULFATES
11
L)
10
J J "Nitrate Selective
ss S
9 ~
,,,," B
8
I i I I I I I I I I I I I i I I
50% SULFATES ......
.
Nitrate Selective .~--- ..........
.s
Nonselective
s
ss"
C
I I I I I I I I I I I I I I I I
20
5 10 15
NaC1, lb/ft 3
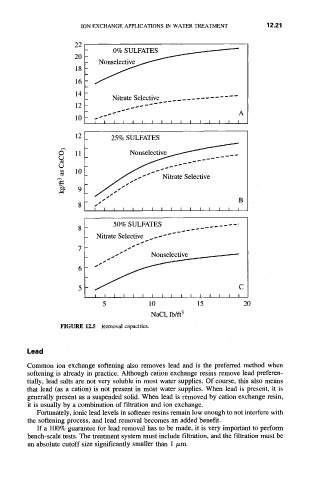
FIGURE 12.5 Removal capacities.
Lead
Common ion exchange softening also removes lead and is the preferred method when
softening is already in practice. Although cation exchange resins remove lead preferen-
tially, lead salts are not very soluble in most water supplies. Of course, this also means
that lead (as a cation) is not present in most water supplies. When lead is present, it is
generally present as a suspended solid. When lead is removed by cation exchange resin,
it is usually by a combination of filtration and ion exchange.
Fortunately, ionic lead levels in softener resins remain low enough to not interfere with
the softening process, and lead removal becomes an added benefit.
If a 100% guarantee for lead removal has to be made, it is very important to perform
bench-scale tests. The treatment system must include filtration, and the filtration must be
an absolute cutoff size significantly smaller than 1/xm.