Page 43 - Hybrid Enhanced Oil Recovery Using Smart Waterflooding
P. 43
CHAPTER 2 Mechanisms of Low-Salinity and Smart Waterflood 35
Total dissolved salts concentration, C TDS (g/L)
1 5 10 50 100
90 0.00
Calcite Surface (R) Calcite Surface (R)
θ w Crude Oil θ w
Crude Oil High lonic Strength Water (W) 0.25
(CO)
Increasing water-wettability Contact angle, w (°) θ 60 0.50 COS w θ
(CO)
Low lonic Strength Water (W)
30 Increasing oil recovery 0.75
0 1.00
0.01 0.1 1 10
[DI water]
[SW]/100 [SW]/20 [SW]/10 [SW]/2 [SW] [FW]
NaCI equivalent concentration, C TDS (mol/L)
Decreasing ionic stregnth
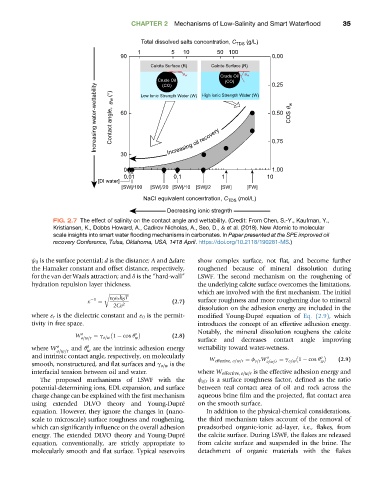
FIG. 2.7 The effect of salinity on the contact angle and wettability. (Credit: From Chen, S.-Y., Kaufman, Y.,
Kristiansen, K., Dobbs Howard, A., Cadirov Nicholas, A., Seo, D., & et al. (2018). New Atomic to molecular
scale insights into smart water flooding mechanisms in carbonates. In Paper presented at the SPE improved oil
recovery Conference, Tulsa, Oklahoma, USA, 1418 April. https://doi.org/10.2118/190281-MS.)
j 0 is the surface potential; d is the distance; A and Ddare show complex surface, not flat, and become further
the Hamaker constant and offset distance, respectively, roughened because of mineral dissolution during
for the van der Waals attraction; and d is the “hard-wall” LSWF. The second mechanism on the roughening of
hydration repulsion layer thickness. the underlying calcite surface overcomes the limitations,
which are involved with the first mechanism. The initial
r ffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffi
ε 0 ε r k B T
k 1 ¼ (2.7) surface roughness and more roughening due to mineral
2Ce 2 dissolution on the adhesion energy are included in the
where ε r is the dielectric constant and ε 0 is the permit- modified Young-Dupré equation of Eq. (2.9),which
tivity in free space. introduces the concept of an effective adhesion energy.
Notably, the mineral dissolution roughens the calcite
o
W o=w=r ¼ g o=w 1 cos q o w (2.8) surface and decreases contact angle improving
o
where W o and q are the intrinsic adhesion energy wettability toward water-wetness.
o=w=r w
and intrinsic contact angle, respectively, on molecularly o o
W effective; o=w=r ¼ f o=r W o=w=r ¼ g o=w 1 cos q w (2.9)
smooth, nonstructured, and flat surfaces and g o/w is the
interfacial tension between oil and water. where W effective, o/w/r is the effective adhesion energy and
The proposed mechanisms of LSWF with the f o/r is a surface roughness factor, defined as the ratio
potential-determining ions, EDL expansion, and surface between real contact area of oil and rock across the
charge change can be explained with the first mechanism aqueous brine film and the projected, flat contact area
using extended DLVO theory and Young-Dupré on the smooth surface.
equation. However, they ignore the changes in (nano- In addition to the physical-chemical considerations,
scale to microscale) surface roughness and roughening, the third mechanism takes account of the removal of
which can significantly influence on the overall adhesion preadsorbed organic-ionic ad-layer, i.e., flakes, from
energy. The extended DLVO theory and Young-Dupré the calcite surface. During LSWF, the flakes are released
equation, conventionally, are strictly appropriate to from calcite surface and suspended in the brine. The
molecularly smooth and flat surface. Typical reservoirs detachment of organic materials with the flakes