Page 103 - A Practical Introduction to Optical Mineralogy
P. 103
SILICATE MINERALS MICA GROUP
Melilite group Sorosilicates monovalent cations K • or Na +, and the perfect mica cleavage occurs
along these planes of K • or Na • ions. Some oxygen ions are replaced by
(Ca,Nah(Mg,Al)(Al,Si),O, tetragonal
hydroxyl ions. Thus the general formula is:
cia = 0.65
This group comprises a series from the Ca,Al end member gehlenite to X,Y.-oZs020(0H,F). with X= K, Na (Ba, Rb, Cs, Ca)
the Ca,Mg end member akermanite. The mineral melilite occupies an Y = Mg,Fe,Al (Mn, Li, Ce, Ti, V, Zn, Co,
intermediate position with Na and Fe'• included as well as Ca, Mg Cu, V)
and AI. Z = Si, AI (Ti, Ge)
n 0 = 1.670 (Geh)-1.632 (Ak) The principal mica group minerals described in detail are phlogopite,
ne = 1.658 (Geh)-1.640 (Ak)
biotite and muscovite. Other micas which may be encountered in rocks
8 = 0.012-0.000 (Geh); 0.000-0.008 (Ak) include lepidolite (Li-bearing, colourless) found in granite pegmatites,
Uniaxial +ve (Geh), -ve (Ak)
paragonite (Na-bearing, colourless) found in some sodium-rich
D = 3.04-2.944 H = 5-6
metamorphic rocks, and glauconite (K, Na and Ca-bearing and iron
coLouR Colourless, variable in browns. rich) - a complex mica, green in colour and pleochroic in greens. It is
PLEOCHROISM Absent from all except thick sections of melilite with o golden brown, e formed as an authigenic mineral in sediments- limestones, sandstones
colourless. and siltstones- and is produced in reducing conditions. All these miner-
*HABIT Melilite group minerals usually appear as subhedral to euhedral crystals, als have properties similar to the micas described (particularly biotite).
tabular on the basal plane.
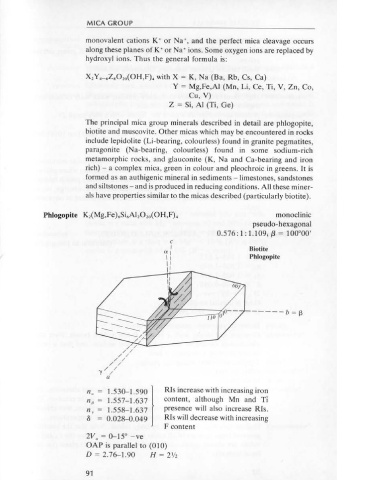
cLEAVAGE {001} moderate, {110} poor. Phlogopite K,(Mg,Fe).Si.Al,0 20 (0H,F) 4 monoclinic
RELIEF Moderate. pseudo-hexagonal
ALTERATION Melilite alters to fibrous masses of cebollite Ca,Al,Si,0, 4 (0H), or 0.576 : 1: 1.109; f3 = 100°00'
prehnite Ca,Al(A!Si,)0, 0 (0H),; the latter reaction is as follows: c
I Biotite
ar
lr Phlogopite
I I
I'
Other minerals like calcite, zeolite, garnet have been reported as altera-
tion products from melilite breakdown.
*BIREFRINGENCE Low tovey low, anomalous interference colours may be seen, with dark
blue instead of first order grey.
INTER FE REN CE Difficult to obtain, with poorly defined isogyres on a pale grey field. ------b=f3
FIGURE
ZONING Oscillatory zoning common.
DISTINGUISHING Very low birefringence and anomalous interference colours are distinc-
FEATURES
tive, as is the limited occurrence. / /
/ /
*OCCURRENCE Melilite occurs in the groundmass of some silica-poor calcium-rich / / / /
/ /
extrusive basalts (melilitites). Melilite may form under high T low P / /
'I /
metamorphism (sanidinite facies) at the contact between silica-poor a
n. = 1.530-1590 1
magmas and carbonate sediments.
Rls increase with increasing iron
np = 1.557-1.637 content, although Mn and Ti
Mica group Phyllosilicates
n y = 1.558-1.637 presence will also increase Rls.
The micas contain sheets of cations such as Fe, Mg or AI (the octahedral 8 = 0.028-0.049 Rls will decrease with increasing
sheets), which are linked to two sheets of linked (Si0 4 ) tetrahedra; the F content
tetrahedral sheets and the mica minerals belong to the 2: 1layer silicates 2V. = 0-15" -ve
because of this ratio of tetrahedral to octahedral sheets. The complete OAP is parallel to (010)
'sandwich unit' is linked to another similar unit by weakly bonded D = 2.76-1.90 H = 2V2
90 91