Page 146 - A Practical Introduction to Optical Mineralogy
P. 146
CARBONATES
THE NON-S ILICATES
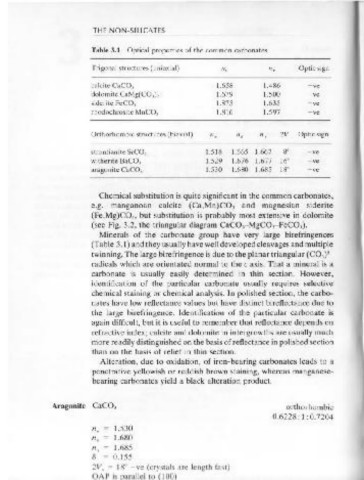
Table 3.1 Optical properties of the common carbonates.
Trigonal structures (uniaxial) no ne Optic sign
c = Cl
calcite CaCO, 1.658 1.486 - ve I
I
dolomite CaMg(CO,), 1.679 1.500 -ve I
siderite FeCO, 1.875 1.635 - ve I
rhodochrosite MnCO, 1.816 1.597 - ve
Orthorhombic structures (biaxial) n. np n y 2V Optic sign
strontianite SrCO, 1.518 1.665 1.667 go - ve
witherite BaCO, 1.529 1.676 1.677 16° - ve
aragonite CaCO, 1.530 1.680 1.685 18° - ve
- ---- -- b ='I
Chemical substitution is quite significant in the common carbonates,
e.g. manganoan calcite (Ca,Mn)CO, and magnesian siderite
(Fe,Mg)CO,, but substitution is probably most extensive in dolomite /
/ 0 10
(see Fig. 3.2, the triangular diagram CaCO,-MgCO,-FeCO,). /
a= j3
Minerals of the carbonate group have very large birefringences
(Table 3.1) and they usually have well developed cleavages and multiple
2
twinning. The large birefringence is due to the planar triangular (C0,) -
radicals which are orientated normal to the c axis. That a mineral is a
carbonate is usually easily determined in thin section. However,
identification of the particular carbonate usually requires selective
COLOU R Colourless. .
chemical staining or chemical analysis. In polished section, the carbo- Thin prismatic or occasionally fibrous crystals occur as for example m
* HABIT
nates have low reflectance values but have distinct bireflectance due to shell structures.
the large birefringence. Identification of the particular carbonate is CLEAVAGE { 010} prismatic cleavage imperfect. . . . .
again difficult, but it is useful to remember that reflectance depends on Low to moderate but variable with opt1c onentatwn, as for calc1te.
refractive index; calcite and dolomite in intergrowths are usually much Minimum RI is parallel to c axis (i.e. parallel to prism length).
RELIEF
more readily distinguished on the basis of reflectance in polished section Extremely high, similar to calcite.
• 01 REFRINGENCE
than on the basis of relief in thin section. Difficult to obtain because of crystal size, but good Bxa figure may be
INTERFERENCE
Alteration, due to oxidation, of iron-bearing carbonates leads to a FIGURE seen on basal section (2V very small).
penetrative yellowish or reddish brown staining, whereas manganese- EXTINCTION Straight on cleavage or prism edge.
·
bearing carbonates yield a black alteration product. TWINN ING Common, arne II ar wms on { 11 0} parallel to c axis. Repeated
I
t
,
twinning also common. . .
Aragonite is Jess common than calcite. Many inv~rtebra~es bu1~d therr
Aragonite CaCO, orthorhombic 0 URRENCE
shells of aragonite, which gradually changes to c.alc1te on d.!agens1s. Th.us
0.6228: 1:0.7204
pre-Mesozoic fossil shells will inevitab~y cons1~t ~f cal~1te. Ar~gom~e
n. = 1.530 occurs as a secondary mineral, often m assoc1at1on w1t~ ze~htes, m
np = 1.680 cavities in volcanic ro k . It is a widespread meta~orph1c m1~eral m
n, = 1.685 lou ophan s hist ra i ·s Ill tumorphi ro ks in Whl h deep bu n al pr -
8 ... 0. 155 du s r on it IN th stuhl ' • 1rhon 11 11 1 ()() • und ) to I 0 kb
11 11
2V, I R• v ( r stu ls ar I n th fus1) on ill l1lvt11 ion to t' lit• 1 111 t o ' 'til' IN th ro •k llllldns
I ll'SN\11 ' ·
0 P Is Pil l ti l I 10 (I 00