Page 151 - A Practical Introduction to Optical Mineralogy
P. 151
THE NON-SILICATES
Sphalerite
Figure3.3 Pyrite
coLouR Colourless to pale brown or pale yellow.
Sulphide Key
HABIT Euhedral crystals are common, and siderite is often found as aggregates
structures (after
of crystals in oolitic structures. Q s
Vaughan &
All other properties similar to calcite (note extreme birefringence).
Craig 1978). e Fe
occuRRENCE Common in ironstone nodules in Carboniferous argillaceous rocks and
also in the Jurassic ironstones of central England. In Raasay in the Inner
Hebrides siderite is associated with chamosite.
Siderite is found in veins with other gangue minerals and metallic
ores.
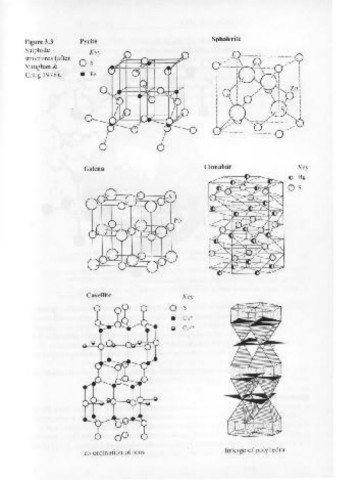
3.3 Sulphides
In the structures of sulphide minerals, sulphur atoms are usually sur- Cinnabar Key
rounded by metallic atoms (e.g. Cu, Zn, Fe) or the semi-metals (Sb, As Galena
() Hg
or Bi). The chemical bonding is usually considered to be essentially
covalent. Although sulphur has a preference for fourfold tetrahedral Qs
co-ordination it is found in a large variety of co-ordination polyhedra
which may be quite asymmetric. Non-stoichiometry, i.e. a variable
metal : sulphur ratio, is a feature of many sulphide structures, especially
at high temperatures; complex ordering may result on cooling of a
non-stoichiometric phase leading, at low temperature, to minerals with
only slightly different compositions but different structures. A good
example is that of high temperature cubic digenite, Cu 2 -xS (x .;;; 0.2),
which is represented at low temperatures by orthorhombic chalcocite
Cu,S, orthorhombic djurleite Cu~, 9 ,S and cubic digenite Cu~, 8 S.
Two further possible complexities in sulphide structures are the exis-
tence of sulphur-sulphur bonds exemplified by the s~- pair in pyrite FeS,
Covellite Key
(see Fig. 3.3), and the existence of structures that can be considered as
resulting from a replacement by a semi-metal of half the sulphur in such 0 s
pairs, e.g. arsenopyrite FeAsS.
Most sulphides are opaque but some (e.g. sphalerite when pure zinc
sulphide) are transparent. Some are transparent for red light (e.g.
pyragyrite Ag 3 SbS 3 ) or only in the infra-red (e.g. stibnite Sb,S 3 ). Many
are semiconductors, which means that they conduct electricity at a high
temperature but not at a low temperature. In fact, the optical and
physical properties of many sulphides are best understood if the band
model of semiconductors is applied (see Shuey 1975).
The structures of several common sulphides are illustrated in Fig-
ure 3.3. As is evident from the few examples given, sulphide structures
can be classified - as are the silicates- into structures based on chains,
sheets, networks and so on. Although such a classification is of less value
than for the silicates, consideration of structures in such a way helps to
explain crystal morphology, cleavage directions etc. of some sulphides.
The sulphosalts are one group of sulphides which are very diverse
chemically and structurally. They contain a semi-metal as well as a metal co-ordination of ions linkage of polyhedra
138