Page 152 - A Practical Introduction to Optical Mineralogy
P. 152
THE NON-SILICATES
SULPHIDES
1
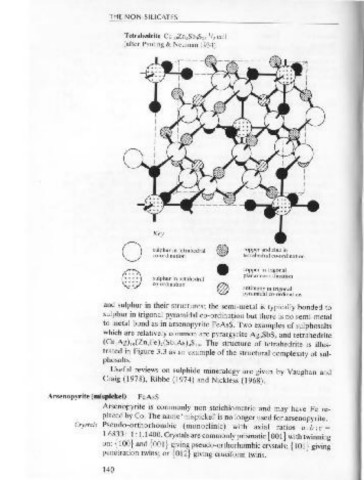
Tetrahedrite Cu 10 Zn 2 Sb 4 S 13 / cell Cleavage { 101} is distinct. D = 6.1.
2
(after Pauling & Neuman 1934)
Polished section Arsenopyrite is white with R = 52 %, about the same as pyrite.
Bireftectance is weak but anisotropy is usually quite distinct, the colours
being dark blues and browns, and extinction is poor. The anisotropy is
easier to observe than that of pyrite but weaker than that of marcasite.
Grain sections are often idiomorphic rhombs or lozenges or rather
elongate skeletal porphyroblasts. Zonation of extinction is common and
simple or hourglass twins are frequently observed. Lamellar twinning is
reported. VHN = 1048- 1127.
Arsenopyrite
rhomb shaped arsenopyrite
grains
[)>---((]
Key
0 sulphur in tetrahedral (@) copper and zinc in PPL
() co-ordination • copper in trigonal Occurrence Arsenopyrite is considered to be typical of relatively high temperature
tetrahedral co-ordin ation
co·ordin ation
.
.
planar co-ordination
sulphur in octahedral
® antim~ny in trigonal hydrothermal veins where cassiterite, wolframite, chalcopyrite, pyrrho-
tite and gold are common associates. It is also found in most types of
pyramtdal co-ordination
sulphide deposits.
and sulphur in their structures; the semi-metal is typically bonded to Distinguishing Compared with arsenopyrite, pyrite is yellowish and cubic in morphol-
sulphur in trigonal pyramidal co-ordination but there is no semi-metal features ogy and marcasite is much more anisotropic.
to metal bond as in arsenopyrite FeAsS·. Two examples of sulphosalts
which are relatively common are pyrargyrite Ag,SbS, and tetrahedrite Bornite Cu,FeS.
(Cu,Ag) ,o(Zn,Fe ),(Sb,As ).S 13 • The structure of tetrahedrite is illus- Crystals Bornite is tetragonal (pseudo-cubic). Crystals are rare as cubes,
trated in Figure 3.3 as an example of the structural complexity of sul- dodecahedra or octahedra. Twinning on { 111} often results in penetra-
phosalts. tion twins. {111} is also a cleavage orientation. D = 5.1.
Useful reviews on sulphide mineralogy are given by Vaughan and Polished section Bornite is pinkish brown when fresh but soon tarnishes to purple or
Craig (1978), Ribbe (1974) and Nickless (1968). iridescent blue. With R = 22 % it is brighter than sphalerite. Both
bireftectance and anisotropy, with dark brown and grey tints, are very
Arsenopyrite (mispickel) FeAsS
weak. Very fine granular aggregates appear isotropic. There is often a
Arsenopyrite is commonly non-stoichiometric and may have Fe re- colour variation or zonation due to tarnishing. Multiple twinning is
placed by Co. The name 'mispickel' is no longer used for arsenopyrite. reported and cleavage traces in two directions are common. Chalcopy-
Crystals Pseudo-orthorhombic (monoclinic) with axial ratios a :b :c = rite is commonly present as myrmekitic intergrowths or lamellae. Chal-
1.6833: 1: 1.1400. Crystals are commonly prismatic [ 001] with twinning copyrite commonly occurs along fractures. Bornite usually occurs as
on: { 10~} and.{ 001} giving pseudo-orthorhombic crystals; { 101} giving granular aggregates but is often intergrown with other Cu + Fe + S
penetration twms; or { 012} giving cruciform twins. minerals. VHN = 97-105.
140
141