Page 179 - A Practical Introduction to Optical Mineralogy
P. 179
THE NON-SILICATES HALIDES
Thin section Spinel is of variable colour and opacity. Mg-rich spinel is transparent
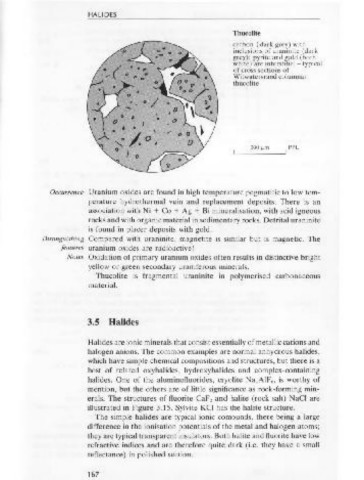
Thucolite
and isotropic. carbon (dark grey) with
Polished Spinel is grey with R = 8%, making it only slightly brighter than associ- ,:--{ ::. inclusions of uraninite (dark
~.
section ated silicates. It is isotropic. Internal reflections vary in abundance . ( .. . grey): pyrite and gold (both
depending on composition. , ... · white) are interstitial - typical
of cross sections of
Spinel is often idiomorphic or rounded octahedral. It may contain Witwatersrand columnar
thucolite
inclusions of magnetite or ilmenite. VHN = 861-1650 spinel;
1402-1561 hercynite.
Occurrence Spinel occurs as exsolved blebs or lamellae in magnetite. It is found in
basic igneous rocks and contact metamorphic and metasomatic alumin- . ,
q •
ous (or Si-deficient) rocks. It is also found as a heavy mineral in placer 0 • I
A. ·/.o~
deposits. Unlike spinel, hercynite is stable in the presence of free silica.
' 0 • I 200 ~m PPL
Uraninite uoz 1 0
Natural uraninite is often oxidised to some extent to pitchblende U0 _ •
2 3
The U is often replaced by Th or Ce.
Crystals Uraninite is cubic and usually occurs as octahedra, cubes or
dodecahedra. Twinning on { 111} is rare. There is no cleavage. D = 9.0. Occurrence Uranium oxides are found in high temperature pegmatitic to low tem-
Thin section Uranium oxides often appear as opaque rounded aggregates altered perature hydrothermal vein and replacement deposits. There is an
along fractures. In thin splinters a green to brown colour may be association with Ni + Co + Ag + Bi mineralisation, with acid igneous
obtained. Associated minerals may be darkened due to radiation dam- rocks and with organic material in sedimentary rocks. Detrital uraninite
age. is found in placer deposits with gold.
Polished Uraninite is grey with R = 17 %, similar to sphalerite. It is cubic and Distinguishing Compared with uraninite, magnetite is similar but is magnetic. The
section isotropic. Pitchblende is similar but slightly darker, with R = 16%. features uranium oxides are radioactive!
Scarce brown internal reflections may be observed in these minerals. Notes Oxidation of primary uranium oxides often results in distinctive bright
Uranium oxides commonly occur as spherical or botryoidal masses. yellow or green secondary uraniferous minerals.
Uraninite is well crystallised but pitchblende varies in crystallinity and Thucolite is fragmental uraninite in polymerised carbonaceous
non-stoichiometry and tends to polish poorly. Composition zoning material.
results in slight brightness and hardness changes. Shrinkage cracks occur
in pitchblende. VHN = 782-839 uraninite; 673-803 pitchblende.
3.5 Halides
Pitchblende
note the 'patchiness' of the Halides are ionic minerals that consist essentially of metallic cations and
brightness due to variation
in oxidation: shrinkage cracks halogen anions. The common examples are normal anhydrous halides,
radiate from the centre of which have simple chemical compositions and structures, but there is a
spheroids
host of related oxyhalides, hydroxyhalides and complex-containing
halides. One of the aluminofluorides, cryolite Na,AlF 6 , is worthy of
mention, but the others are of little significance as rock-forming min-
erals. The structures of fluorite CaF 2 and halite (rock salt) NaCl are
illustrated in Figure 3.15. Sylvite KCI has the halite structure.
The simple halides are typical ionic comounds, there being a large
difference in the ionisation potentials of the metal and halogen atoms;
500 ~m PPL they are typical transparent insulators. Both halite and fluorite have low
refractive indices and are therefore quite dark (i.e. they have a small
reflectance) in polished section.
166
167