Page 180 - A Practical Introduction to Optical Mineralogy
P. 180
THE NON-SILICATES HYDROXIDES
(a) late stage pneumatolytic deposits fluorite occurs with cassiterite, topaz,
Key apatite and lepidolite, whereas in hydrothermal veins fluorite occurs
/ / / with calcite, quartz, barite and sulphides.
/ / / 0 Cl
Fluorite is occasionally found as the cementing matrix in sandstones
e Na
and may occur in geodes within limestones. Blue John is coarse nodular
purple fluorite with a concentric layered structure.
/ / /I-
/ ./ /
Halite (rock NaCl cubic
salt) n = 1.544
/ / ./ D = 2.16 H = 2112
/ / / -
Halite is colourless rock salt with a perfect { 100} cubic cleavage. It
occurs in salt domes and in evaporates, where it is a late precipitating
salt.
(b) Key
Note: Special sectioning techniques are needed to preserve this mineral
e Ca in thin sections.
~ ---- - "7",
/ I 0 F
I
I
I
I
I
I
I 3.6 Hydroxides
___ v I
Brucite Mg(OH), trigonal, cia 1.521
1.560-1.590
n 0
n. 1.580-1.600
0 0.012-0.020
Uniaxial +ve (length fast)
D = 2.4 H = 2%
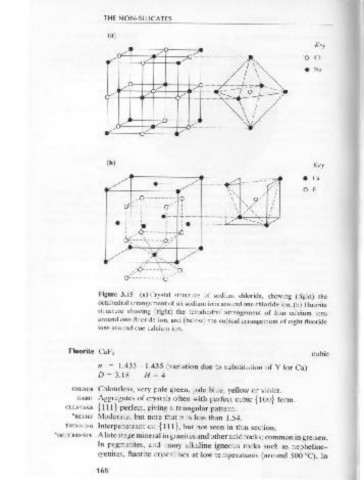
Figure 3.15 (a) Crystal structure of sodium chloride, showing (right) the coLOuR Colourless.
octahedral arrangement of six sodium ions around one chloride ion. (b) Fluorite
HABIT Occurs as fine aggregates, or fibrous whorls, in metamorphosed impure
structure showing (right) the tetrahedral arrangement of four calcium ions
limestones.
around one fluoride ion, and (below) the cubical arrangement of eight fluoride
ions around one calcium ion. *cLEAVAGE Perfect basal {0001}.
RELIEF Low, just greater than 1.54.
ALTERATION Brucite forms from periclase MgO by addition of H,O during thermal
Fluorite CaF,
cubic metamorphism. It alters to hydromagnesite readily by reaction with
carbon dioxide:
n = 1.433 - 1.435 (variation due to substitution of Y for Ca)
D = 3.18 H = 4
5Mg(OH), + 4C0 2 ~ Mg,(OH),(C0,) • .4H,O
COLOUR Colourless, very pale green, pale blue, yellow or violet. hydromagnesite
HABIT Aggregates of crystals often with perfect cubic { 100} form.
CLEAVAGE { 111} perfect, giving a triangular pattern. *BIREFRINGENCE Low, first order colours, but often shows anomalous interference col-
*RELIEF Moderate, but note that n is less than 1.54. ours (deep blue) rather similar to chlorite.
TWINNING Interpenetrant on { 111}, but not seen in thin section. TWINNING None.
*occuRRENCE A late stage mineral in granites and other acid rocks; common in greisen. *occuRRENCE Brucite occurs in thermally metamorphosed dolomites, and dolomitic
In pegmatites, and many alkaline igneous rocks such as nepheline- limestones. It can occur in low temperature hydrothermal veins, associ-
syenites, fluorite crystallises at low temperatures (around 500 °C). In ated with serpentinites and chlorite schists.
168
169