Page 67 - A Practical Introduction to Optical Mineralogy
P. 67
SILICATE MINERALS AMPHIBOLE GROUP
TWINNING Simple or repeated on { 100}. Note that the mineral barkevikite is no longer recognised as a distinct
•occuRREN CE Rare: formed in metamorphic skarns and in thermally metamorphosed mineral and the name has been formally abandoned. Barkevikite was a
limestones. name used to describe an iron-rich pargasitic hornblende, and was never
chemically defined (Leake 1978).
The following monoclinic amphiboles are also brown in colour:
2
tlllllllltll • urfvedsonite Na 2 Na(Mg,Fe •).AISi 80 n(OH,F)2 monoclinic
3
Katophorite Na2Ca(Mg,Fe).Fe •(Si7AJ)0 22 (0H) 2
3
Oxyhornblende NaCa 2 (Mg,Fe,Fe •,Ti,AJ),(Si 6 A1 2 )0 22 (0,0H) 2
Cl
(basaltic hornblende) I
Kaersutite (Na,K)Ca 2 (Mg,Fe ) 4 Ti(Si 6 Al 2 )0 2 ,( 0 H), I
010
c oLouR Oxyhornblende and kaersutite are dark brown in colour.
PLEOCHROISM Oxyhornblende, 0! yellow, {3 and 'Y dark brown. Kaersutite, a yellowish,
{3 reddish brown, y dark brownish. Katophorite is strongly coloured in
yellows, browns or greens, with a yellow or pale brown, {3 greenish
brown or dark brown, and y greenish brown, red brown or purplish
brown. In iron-rich varieties {3 andy become more greenish andy may
be black. ---- b =(3
INTERFERENCE All minerals are negative with 2V. of:
FIGURE
0-50° katophorite
0- 0o { oxyhor~blende
6 8
kaersuttte
EXTINCTION Extinction angles measured on an (010) section vary with composition,
ANGLE as follows:
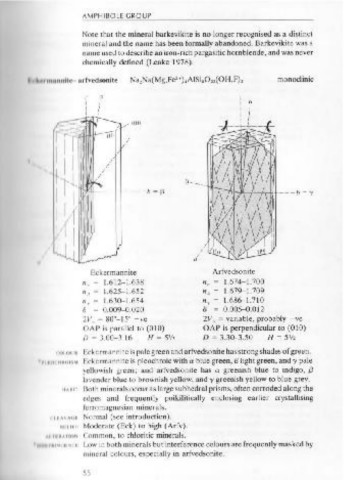
ckermannite Arfvedsonite
.ffcleavage zoo to 54° katophorite /1 .. 1.612-1.638 n. = 1.674-1.700
oxyhornblende " " = 1.625-1.652 n p = 1.679-1.709
y"cleavage 0 to 19o { kaersutite 11 y = 1.630-1.654 n, = 1.686-1.710
fJ = 0.009-0.020 a = o.oo5- 0.012
2V. = 80°-15° - ve 2V. = variable, probably -ve
SUMMARY OF Katophorite is very strongly coloured and pleochroic in yellows, browns
PROPERTIES and greens, and with 2V. variable (0-50°) and a large extinction angle AP is parallel to (010) OAP is perpendicular to (010)
.ffcl = 20 to 54° on an (01 0) section. Note that the OAP is perpendicular I) = 3.00-3.16 H = SV2 D = 3.30-3.50 H = Slf2
to (010). I IIIIIII K .ckermannite is pale green and arfvedsonite has strong shades of green.
Oxyhornblende is pleochroic in yellows and dark browns, and with '11"111 M bckermannite is pleochroic with a blue green, {3 light green, andy pale
2V. large and with a small angle y"cl = 0 to 19' on an (010) section. yellowish green ; and arfvedsonite has a greenish blue to indigo, {3
Kaersutite is pleochroic in yellows and reddish browns, and with 2V. lavender blue to brownish yellow, andy greenish yellow to blue grey.
large. Extinction angles are small with y"cl 0 to 19° on an (01 0) section. II 1111 Both minerals occur as large subhedral prisms, often corroded along the
dges and frequently poikilitically enclosing earlier crystallising
OCCURRENCE Katophorite occurs in dark coloured alkali intrusives in association with f rr magnesian minerals.
nepheline, aegirine and arfvedsonite. Kaersutite occurs in alkaline vol- Ill ~ I I Normal (see introduction).
canic rocks, and as phenocrysts in trachytes and other K-rich extrusives; • 1 Ill I Moderate (Eck) to high (Arfv).
and it may be present in some monzonites. 'ommon, to ch!oritic minerals.
Oxyhornblende occurs mainly as phenocrysts in intermediate vol- I w in both minerals but interference colours are frequently masked by
canic or hypabyssal rocks such as andesites, trachytes and so on. mineral colours, especially in arfvedsonite.
54