Page 87 - A Practical Introduction to Optical Mineralogy
P. 87
SILICATE MINERALS FELDSPAR GROUP
Low microcline
showing cross-hatched twinning
600fLm
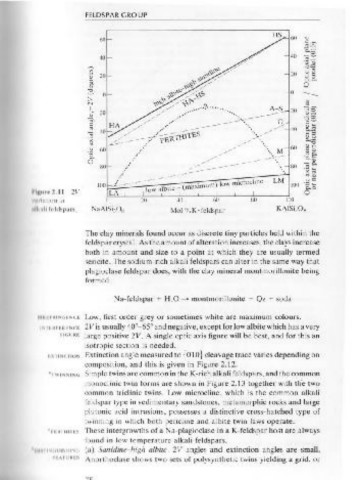
There is a continuous variation in RI from K- to Na-alkali feldspar, and
general values for the end members are as follows:
Ab Or
na 1.527 1.518
I urt~2. 11 2V
n/3 1.531 1.522
Ill IliOn in
ny 1.539 1.524
I 1111 ltlspars. Mol % K-feldspar
a 0.012 0.006
2V is variable in size or sign depending on composition and tyfll' , The clay minerals found occur as discrete tiny particles held within the
Figure 2.11 gives the full range of values. 2V values are 15°-4()" feldspar crystal. As the amount of alteration increases, the clays increase
sanidine, 42°-52° anorthoclase and 52°-54° high albite; all are biaxi11l both in amount and size to a point at which they are usually termed
negative, as is orthoclase (35°-50°) and microcline (66°-90°). Low alhilt• sericite. The sodium-rich alkali feldspars can alter in the same way that
is biaxial positive with 2V, = 84°-78° plagioclase feldspar does, with the clay mineral montmorillonite being
OAP also varies; this variation is given in Figure 2.11 and in the variou ~ formed
feldspar diagrams
D = 2.56-2.63 H = 6-61!2 Na-feldspar + H 2 0-+ montmorillonite + Qz + soda
COLOU R Colourless with opaque patches if alteration to clay minerals Ill!
"II "INioi· N E Low, first order grey or sometimes white are maximum colours.
occurred.
II'! II "II KI•NCE 2V is usually 40°-65° and negative, except for low albite which has a very
HABIT Euhedral prismatic in high temperature porphyritic rocks to anhedrul111
II(,IJ RE large positive 2V. A single optic axis figure will be best, and for this an
plutonic intrusive rocks, although some porphyritic granites con111111
isotropic section is needed.
euhedral (high T) alkali feldspar phenocrysts; for example the gran1t1
from Shap Fell, Cumbria. I IINI liON Extinction angle measured to { 010} cleavage trace varies depending on
composition, and this is given in Figure 2.12.
CLEAVAGE Two cleavages { 001} and { 010} meeting at. nearly right angle on lin
!WINN ING imple twins are common in the K-rich alkali feldspars, and the common
(100) plane. Several partings may be present.
monoclinic twin forms are shown in Figure 2.13 together with the two
RELIEF Low, less than 1.54.
common triclinic twins. Low microcline, which is the common alkali
* ALTERATION Common, usually to clay minerals with K-feldspar altering as foll ow~ 111
feldspar type in sedimentary sandstones, metamorphic rocks and large
the presence of a limited amount of water:
plutonic acid intrusions, possesses a distinctive cross-hatched type of
3 Or + 2 H 20-+ illite + 6 silica + 2 potash !winning in which both pericline and albite twin laws operate.
•t'IH IIII II ~ These intergrowths of aNa-plagioclase in a K-feldspar host are always
or if excess water is present, then found in low temperature alkali feldspars.
II 111111111 II IN<• (a) anidine-high albite. 2 V angles and extinction angles are small.
2 r I kaolin 1 4 silica 1 2 potash II 111M" Anorlho lase hows two ets of polysynthetic twins yielding a grid, or