Page 42 - [B._MURPHY,_C._MURPHY,_B._HATHAWAY]_A_working_meth
P. 42
26 Chapter 3
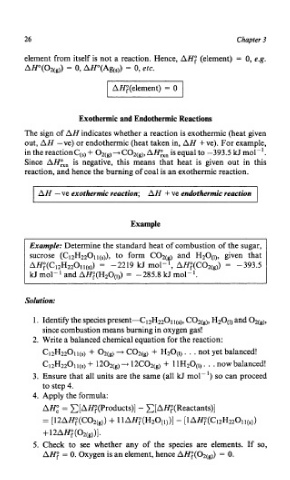
element from itself is not a reaction. Hence, AH; (element) = 0, e.g.
AH0(02(g)) = 0, AHo(Ag(s)) = 0, etc.
I AH;(element) = 0 I
Exothermic and Endothermic Reactions
The sign of AH indicates whether a reaction is exothermic (heat given
out, AH -ve) or endothermic (heat taken in, AH + ve). For example,
in the reactionC(,) + 02(g)-+ CO,,,), AH:x, is equal to -393.5 kJ mol-'.
Since AH;, is negative, this means that heat is given out in this
reaction, and hence the burning of coal is an exothermic reaction.
I AH - ve exothermic reaction; AH + ve endothermic reaction I
Example
Example: Determine the standard heat of combustion of the sugar,
sucrose (C12H2201 I(s)), to form C02(g) and H20(1), given that
AHF(C12H22011(s)) = -2219 kJ mol-', AH;(C02(g)) = -393.5
kJ mol- and AHF(H20(1)) = - 285.8 kJ mol-'.
Solution:
1. Identify the species ~resent4~2H~~O~
C02(g), H,O(l) and 02(g),
since combustion means burning in oxygen gas!
2. Write a balanced chemical equation for the reaction:
C12H22011(s) + 02(g, -+ C02(,) + H200) . . . not yet balanced!
l(s) + 1202(g, --+ 12C02(g) + 1 1H20(,) . . . now balanced!
C12H2201
3. Ensure that all units are the same (all kJ mol-') so can proceed
to step 4.
4. Apply the formula:
AH; = C[AH;(Products)] - C[AHF(Reactants)]
= [12AH;(c02(g)) + llnH;(H20(1))] - [lAHfO(C12H22011(s))
+ 12Af-q (02(g) 11 *
5. Check to see whether any of the species are elements. If so,
AH; = 0. Oxygen is an element, hence AH;(OZ(~)) = 0.