Page 37 - [B._MURPHY,_C._MURPHY,_B._HATHAWAY]_A_working_meth
P. 37
Thermodynamics I 21
i.e. the change in enthalpy is independent of the path followed. For this
reason, enthalpy, like internal energy, is also a state function, a
quantity whose value is determined only by the state of the system in
question.
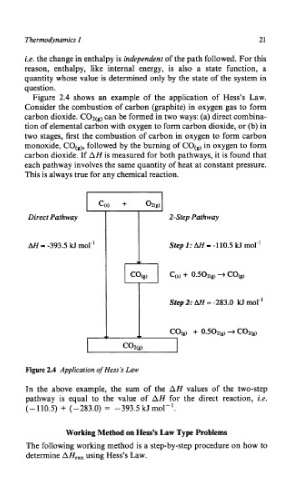
Figure 2.4 shows an example of the application of Hess’s Law.
Consider the combustion of carbon (graphite) in oxygen gas to form
carbon dioxide. COz(,, can be formed in two ways: (a) direct combina-
tion of elemental carbon with oxygen to form carbon dioxide, or (b) in
two stages, first the combustion of carbon in oxygen to form carbon
monoxide, CO,,), followed by the burning of CO,,) in oxygen to form
carbon dioxide. If AH is measured for both pathways, it is found that
each pathway involves the same quantity of heat at constant pressure.
This is always true for any chemical reaction.
Direct Pathway 2-Step Pathway
AH - -393.5 H mol-’ Step I: AH = -1 10.5 kl mol-’
Figure 2.4 Application of Hem’s Law
In the above example, the sum of the AH values of the two-step
pathway is equal to the value of AH for the direct reaction, i.e.
(- 110.5) + (-283.0) = -393.5 kJ mol-*.
Working Method on Hess’s Law Type Problems
The following working method is a step-by-step procedure on how to
determine AH,,, using Hess’s Law.