Page 68 - [B._MURPHY,_C._MURPHY,_B._HATHAWAY]_A_working_meth
P. 68
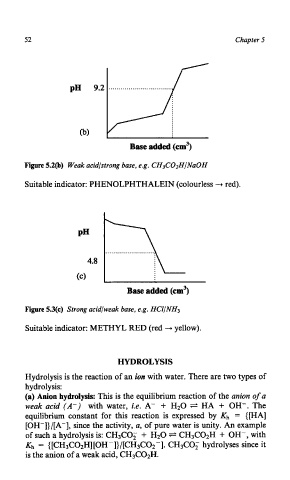
52 Chapter 5
pH 9.2
cb)
Base added (cm3)
Figure 5.2@) Weak acidlstrong base, e.g. CH3C02HINaOH
Suitable indicator: PHENOLPHTHALEIN (colourless + red).
Base added (cm3)
Figure 5.3(c) Strong acidlweak base, e.g. HCIINH3
Suitable indicator: METHYL RED (red + yellow).
HYDROLYSIS
Hydrolysis is the reaction of an ion with water. There are two types of
hydrolysis:
(a) Anion hydrolysis: This is the equilibrium reaction of the anion of a
weak acid (A-) with water, i.e. A- + H20 + HA + OH-. The
equilibrium constant for this reaction is expressed by Kh = {[HA]
[OH-])/[A-], since the activity, a, of pure water is unity. An example
of such a hydrolysis is: CH3CO: + H20 + CH3C02H + OH-, with
Kh = { [CH3C02H] [OH-]}/[CH3C02-]. CH3CO; hydrolyses since it
is the anion of a weak acid, CH3C02H.