Page 247 - Adsorbents fundamentals and applications
P. 247
232 CARBON NANOTUBES, PILLARED CLAYS, AND POLYMERIC RESINS
(a)
(b)
(c)
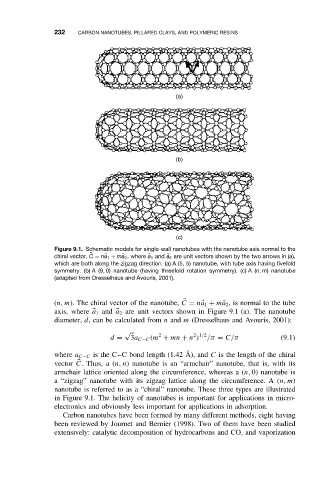
Figure 9.1. Schematic models for single-wall nanotubes with the nanotube axis normal to the
chiral vector, C = n a 1 + m a 2 ,where a 1 and a 2 are unit vectors shown by the two arrows in (a),
which are both along the zigzag direction. (a) A (5, 5) nanotube, with tube axis having fivefold
symmetry. (b) A (9, 0) nanotube (having threefold rotation symmetry). (c) A (n, m)nanotube
(adapted from Dresselhaus and Avouris, 2001).
a
a
(n, m). The chiral vector of the nanotube, C = n 1 + m 2 , is normal to the tube
a
axis, where 1 and a 2 are unit vectors shown in Figure 9.1 (a). The nanotube
diameter, d, can be calculated from n and m (Dresselhaus and Avouris, 2001):
√
2
2 1/2
d = 3a C−C (m + mn + n ) /π = C/π (9.1)
where a C−C is the C–C bond length (1.42 ˚ A), and C is the length of the chiral
vector C. Thus, a (n, n) nanotube is an “armchair” nanotube, that is, with its
armchair lattice oriented along the circumference, whereas a (n, 0) nanotube is
a “zigzag” nanotube with its zigzag lattice along the circumference. A (n, m)
nanotube is referred to as a “chiral” nanotube. These three types are illustrated
in Figure 9.1. The helicity of nanotubes is important for applications in micro-
electronics and obviously less important for applications in adsorption.
Carbon nanotubes have been formed by many different methods, eight having
been reviewed by Journet and Bernier (1998). Two of them have been studied
extensively: catalytic decomposition of hydrocarbons and CO, and vaporization