Page 252 - Adsorbents fundamentals and applications
P. 252
CARBON NANOTUBES 237
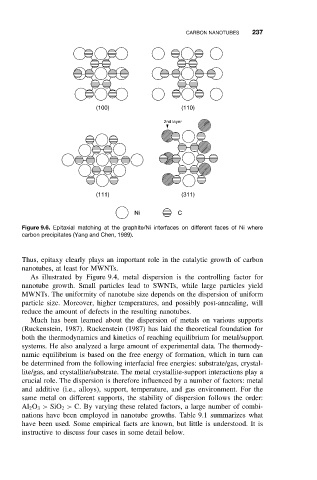
(100) (110)
2nd layer
(111) (311)
Ni C
Figure 9.6. Epitaxial matching at the graphite/Ni interfaces on different faces of Ni where
carbon precipitates (Yang and Chen, 1989).
Thus, epitaxy clearly plays an important role in the catalytic growth of carbon
nanotubes, at least for MWNTs.
As illustrated by Figure 9.4, metal dispersion is the controlling factor for
nanotube growth. Small particles lead to SWNTs, while large particles yield
MWNTs. The uniformity of nanotube size depends on the dispersion of uniform
particle size. Moreover, higher temperatures, and possibly post-annealing, will
reduce the amount of defects in the resulting nanotubes.
Much has been learned about the dispersion of metals on various supports
(Ruckenstein, 1987). Ruckenstein (1987) has laid the theoretical foundation for
both the thermodynamics and kinetics of reaching equilibrium for metal/support
systems. He also analyzed a large amount of experimental data. The thermody-
namic equilibrium is based on the free energy of formation, which in turn can
be determined from the following interfacial free energies: substrate/gas, crystal-
lite/gas, and crystallite/substrate. The metal crystallite-support interactions play a
crucial role. The dispersion is therefore influenced by a number of factors: metal
and additive (i.e., alloys), support, temperature, and gas environment. For the
same metal on different supports, the stability of dispersion follows the order:
Al 2 O 3 > SiO 2 > C. By varying these related factors, a large number of combi-
nations have been employed in nanotube growths. Table 9.1 summarizes what
have been used. Some empirical facts are known, but little is understood. It is
instructive to discuss four cases in some detail below.