Page 27 - Adsorbents fundamentals and applications
P. 27
12 FUNDAMENTAL FACTORS FOR DESIGNING ADSORBENT
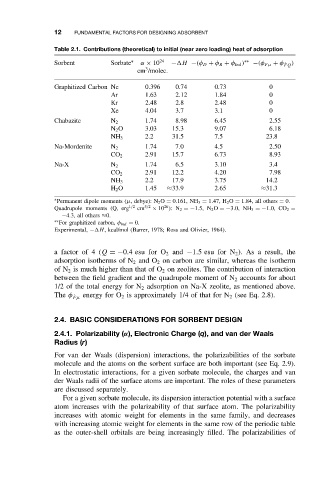
Table 2.1. Contributions (theoretical) to initial (near zero loading) heat of adsorption
Sorbent Sorbate ∗ α × 10 24 − H −(φ D + φ R + φ Ind ) ∗∗ −(φ Fµ + φ ˙ FQ )
3
cm /molec.
Graphitized Carbon Ne 0.396 0.74 0.73 0
Ar 1.63 2.12 1.84 0
Kr 2.48 2.8 2.48 0
Xe 4.04 3.7 3.1 0
Chabazite N 2 1.74 8.98 6.45 2.55
N 2 O 3.03 15.3 9.07 6.18
2.2 31.5 7.5 23.8
NH 3
Na-Mordenite N 2 1.74 7.0 4.5 2.50
CO 2 2.91 15.7 6.73 8.93
Na-X N 2 1.74 6.5 3.10 3.4
CO 2 2.91 12.2 4.20 7.98
NH 3 2.2 17.9 3.75 14.2
H 2 O 1.45 ≈33.9 2.65 ≈31.3
∗ Permanent dipole moments (µ, debye): N 2 O = 0.161, NH 3 = 1.47, H 2 O = 1.84, all others = 0.
26
Quadrupole moments (Q, erg 1/2 cm 5/2 × 10 ): N 2 =−1.5, N 2 O =−3.0, NH 3 =−1.0, CO 2 =
−4.3, all others ≈0.
∗∗ For graphitized carbon, φ Ind = 0.
Experimental, − H, kcal/mol (Barrer, 1978; Ross and Olivier, 1964).
a factor of 4 (Q =−0.4esu for O 2 and −1.5esu for N 2 ). As a result, the
adsorption isotherms of N 2 and O 2 on carbon are similar, whereas the isotherm
of N 2 is much higher than that of O 2 on zeolites. The contribution of interaction
between the field gradient and the quadrupole moment of N 2 accounts for about
1/2 of the total energy for N 2 adsorption on Na-X zeolite, as mentioned above.
energy for O 2 is approximately 1/4 of that for N 2 (see Eq. 2.8).
The φ ˙ Fµ
2.4. BASIC CONSIDERATIONS FOR SORBENT DESIGN
2.4.1. Polarizability (α), Electronic Charge (q), and van der Waals
Radius (r)
For van der Waals (dispersion) interactions, the polarizabilities of the sorbate
molecule and the atoms on the sorbent surface are both important (see Eq. 2.9).
In electrostatic interactions, for a given sorbate molecule, the charges and van
der Waals radii of the surface atoms are important. The roles of these parameters
are discussed separately.
For a given sorbate molecule, its dispersion interaction potential with a surface
atom increases with the polarizability of that surface atom. The polarizability
increases with atomic weight for elements in the same family, and decreases
with increasing atomic weight for elements in the same row of the periodic table
as the outer-shell orbitals are being increasingly filled. The polarizabilities of